Fibronectin Fragments and Inflammation During Canine Intervertebral Disc Disease
- PMID: 33304936
- PMCID: PMC7701143
- DOI: 10.3389/fvets.2020.547644
Fibronectin Fragments and Inflammation During Canine Intervertebral Disc Disease
Abstract
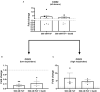
Background: Canine intervertebral disc disease (IVDD) represents a significant clinical problem in veterinary medicine, with similarities to the human pathology. Host-derived damage-associated molecular patterns like fibronectin fragments (FnF) that develop during tissue dysfunction may be of specific relevance to IVD pathologies by inducing an inflammatory response in resident cells. Aim: This project aimed to determine the presence and pathobiological role of FnF during IVD herniation in dogs, with a focus on inflammation. Methods: Herniated nucleus pulposus (NP) material from five dogs as well as non-herniated adjacent NP material from three dogs was collected during spinal surgery required due to acute IVD herniation. The presence of different types of FnF were determined by Western blot analysis. NP cells isolated from six herniated canine IVDs were then exposed to 30 kDa FnF. NP cell inflammation and catabolism was examined by investigating the expression of IL-1β, IL-6, IL-8, and COX-2, as well as MMP-1 and MMP-3 by qPCR (all targets) and ELISA (IL-6, PGE2). Results: Amongst multiple sized FnF (30, 35, 45, and >170kDa), N-terminal fragments at a size of ~30 kDa were most consistently expressed in all five herniated IVDs. Importantly, these fragments were exclusively present in herniated, but not in non-herniated IVDs. Exposure of canine NP cells to 500 nM 30 kDa FnF caused a significant upregulation of IL-6 (62.5 ± 79.9, p = 0.032) and IL-8 (53.0 ± 75.7, p = 0.031) on the gene level, whereas IL-6 protein analysis was inconclusive. Donor-donor variation was observed in response to FnF treatment, whereby this phenomenon was most evident for COX-2, with three donors demonstrating a significant downregulation (0.67 ± 0.03, p = 0.003) and three donors showing upregulation (6.9 ± 5.5, p = 0.21). Co-treatment with Sparstolonin B, a TRL-2/TRL-4 antagonist, showed no statistical difference to FnF treatment alone in all tested target genes. Conclusion: Given the presence of the 30 kDa FnF in canine herniated IVDs and the proinflammatory effect of 30 kDa FnF on NP cells, we concluded that the accumulation of FnF may be involved in the pathogenesis of canine IVDD. These results correspond to the findings in humans with IVDD.
Keywords: DAMPs; IVDD; NP cells; fibronectin fragments; intervertebral disc; neuroinflammation.
Copyright © 2020 Schmidli, Sadowska, Cvitas, Gantenbein, Lischer, Forterre, Hitzl, Forterre and Wuertz-Kozak.
Figures

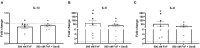

References
-
- Modic MT. Degenerative disc disease and back pain. Magn Reson Imaging Clin N Am. (1999) 7:481–91. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

