AUTS2 Governs Cerebellar Development, Purkinje Cell Maturation, Motor Function and Social Communication
- PMID: 33305180
- PMCID: PMC7708818
- DOI: 10.1016/j.isci.2020.101820
AUTS2 Governs Cerebellar Development, Purkinje Cell Maturation, Motor Function and Social Communication
Abstract
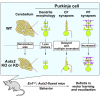
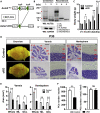
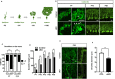
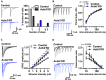
Autism susceptibility candidate 2 (AUTS2), a risk gene for autism spectrum disorders (ASDs), is implicated in telencephalon development. Because AUTS2 is also expressed in the cerebellum where defects have been linked to ASDs, we investigated AUTS2 functions in the cerebellum. AUTS2 is specifically localized in Purkinje cells (PCs) and Golgi cells during postnatal development. Auts2 conditional knockout (cKO) mice exhibited smaller and deformed cerebella containing immature-shaped PCs with reduced expression of Cacna1a. Auts2 cKO and knock-down experiments implicated AUTS2 participation in elimination and translocation of climbing fiber synapses and restriction of parallel fiber synapse numbers. Auts2 cKO mice exhibited behavioral impairments in motor learning and vocal communications. Because Cacna1a is known to regulate synapse development in PCs, it suggests that AUTS2 is required for PC maturation to elicit normal development of PC synapses and thus the impairment of AUTS2 may cause cerebellar dysfunction related to psychiatric illnesses such as ASDs.
Keywords: Cellular Neuroscience; Developmental Neuroscience; Molecular Neuroscience.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Aiba A., Kano M., Chen C., Stanton M.E., Fox G.D., Herrup K., Zwingman T.A., Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J. Comp. Neurol. 1972;145:399–463. - PubMed
-
- Altman J., Bayer S.A. Prenatal development of the cerebellar system in the rat. II. Cytogenesis and histogenesis of the inferior olive, pontine gray, and the precerebellar reticular nuclei. J. Comp. Neurol. 1978;179:49–75. - PubMed
-
- Amaral D.G., Schumann C.M., Nordahl C.W. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

