PET-MRI nanoparticles imaging of blood-brain barrier damage and modulation after stroke reperfusion
- PMID: 33305265
- PMCID: PMC7716090
- DOI: 10.1093/braincomms/fcaa193
PET-MRI nanoparticles imaging of blood-brain barrier damage and modulation after stroke reperfusion
Abstract
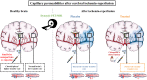
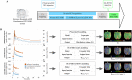
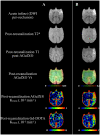
In an acute ischaemic stroke, understanding the dynamics of blood-brain barrier injury is of particular importance for the prevention of symptomatic haemorrhagic transformation. However, the available techniques assessing blood-brain barrier permeability are not quantitative and are little used in the context of acute reperfusion therapy. Nanoparticles cross the healthy or impaired blood-brain barrier through combined passive and active processes. Imaging and quantifying their transfer rate could better characterize blood-brain barrier damage and refine the delivery of neuroprotective agents. We previously developed an original endovascular stroke model of acute ischaemic stroke treated by mechanical thrombectomy followed by positron emission tomography-magnetic resonance imaging. Cerebral capillary permeability was quantified for two molecule sizes: small clinical gadolinium Gd-DOTA (<1 nm) and AGuIX® nanoparticles (∼5 nm) used for brain theranostics. On dynamic contrast-enhanced magnetic resonance imaging, the baseline transfer constant K trans was 0.94 [0.48, 1.72] and 0.16 [0.08, 0.33] ×10-3 min-1, respectively, in the normal brain parenchyma, consistent with their respective sizes, and 1.90 [1.23, 3.95] and 2.86 [1.39, 4.52] ×10-3 min-1 in choroid plexus, confirming higher permeability than brain parenchyma. At early reperfusion, K trans for both Gd-DOTA and AGuIX® nanoparticles was significantly higher within the ischaemic area compared to the contralateral hemisphere; 2.23 [1.17, 4.13] and 0.82 [0.46, 1.87] ×10-3 min-1 for Gd-DOTA and AGuIX® nanoparticles, respectively. With AGuIX® nanoparticles, K trans also increased within the ischaemic growth areas, suggesting added value for AGuIX®. Finally, K trans was significantly lower in both the lesion and the choroid plexus in a drug-treated group (ciclosporin A, n = 7) compared to placebo (n = 5). K trans quantification with AGuIX® nanoparticles can monitor early blood-brain barrier damage and treatment effect in ischaemic stroke after reperfusion.
Keywords: blood–brain barrier; choroid plexus; ischaemia–reperfusion damage; nanoparticles; stroke.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


Similar articles
-
Selective brain entry of lipid nanoparticles in haemorrhagic stroke is linked to biphasic blood-brain barrier disruption.Theranostics. 2022 May 26;12(10):4477-4497. doi: 10.7150/thno.72167. eCollection 2022. Theranostics. 2022. PMID: 35832077 Free PMC article.
-
Evaluation of a Gadolinium-Based Nanoparticle (AGuIX) for Contrast-Enhanced MRI of the Liver in a Rat Model of Hepatic Colorectal Cancer Metastases at 9.4 Tesla.Rofo. 2015 Dec;187(12):1108-15. doi: 10.1055/s-0035-1553500. Epub 2015 Sep 11. Rofo. 2015. PMID: 26361379
-
Identification of variations in blood-brain barrier opening after cerebral ischemia by dual contrast-enhanced magnetic resonance imaging and T 1sat measurements.Stroke. 2008 Feb;39(2):427-32. doi: 10.1161/STROKEAHA.107.496059. Epub 2008 Jan 3. Stroke. 2008. PMID: 18174480
-
EPR-mediated tumor targeting using ultrasmall-hybrid nanoparticles: From animal to human with theranostic AGuIX nanoparticles.Theranostics. 2020 Jan 1;10(3):1319-1331. doi: 10.7150/thno.37543. eCollection 2020. Theranostics. 2020. PMID: 31938067 Free PMC article. Review.
-
Imaging After Thrombolysis and Thrombectomy: Rationale, Modalities and Management Implications.Curr Neurol Neurosci Rep. 2019 Jul 6;19(8):57. doi: 10.1007/s11910-019-0970-7. Curr Neurol Neurosci Rep. 2019. PMID: 31278596 Review.
Cited by
-
Spatio-Temporal Characterization of Brain Inflammation in a Non-human Primate Stroke Model Mimicking Endovascular Thrombectomy.Neurotherapeutics. 2023 Apr;20(3):789-802. doi: 10.1007/s13311-023-01368-2. Epub 2023 Mar 28. Neurotherapeutics. 2023. PMID: 36976495 Free PMC article. Review.
-
Quantitative imaging outperforms No-reflow in predicting functional outcomes in a translational stroke model.Neurotherapeutics. 2025 Mar;22(2):e00529. doi: 10.1016/j.neurot.2025.e00529. Epub 2025 Jan 31. Neurotherapeutics. 2025. PMID: 39893086 Free PMC article.
-
Recent progress on nanotechnologies for enhancing blood-brain barrier permeability.Smart Mol. 2025 Mar 20;3(2):e20240052. doi: 10.1002/smo.20240052. eCollection 2025 Jun. Smart Mol. 2025. PMID: 40671921 Free PMC article. Review.
-
Microglial phagocytosis dysfunction in stroke is driven by energy depletion and induction of autophagy.Autophagy. 2023 Jul;19(7):1952-1981. doi: 10.1080/15548627.2023.2165313. Epub 2023 Jan 20. Autophagy. 2023. PMID: 36622892 Free PMC article.
-
Nanotechnology in Stroke: New Trails with Smaller Scales.Biomedicines. 2023 Mar 4;11(3):780. doi: 10.3390/biomedicines11030780. Biomedicines. 2023. PMID: 36979759 Free PMC article. Review.
References
-
- Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: New insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke 2015; 10: 143–52. - PubMed
-
- Ballanger B, Tremblay L, Sgambato-Faure V, Beaudoin-Gobert M, Lavenne F, Le Bars D, et al. A multi-atlas based method for automated anatomical Macaca fascicularis brain MRI segmentation and PET kinetic extraction. Neuroimage 2013; 77: 26–43. - PubMed
-
- Basseville A, Hall MD, Chau CH, Robey RW, Gottesman M, Figg WD, et al. The ABCG2 multidrug transporter In: George AM, editor. ABC transporters - 40 years on. Cham: Springer International Publishing; 2016. p. 195–226.
-
- Broocks G, Hanning U, Flottmann F, Schönfeld M, Faizy TD, Sporns P, et al. Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by oedema reduction. Brain 2019; 142: 1399–407. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
