OnabotulinumtoxinA for the treatment of neurogenic detrusor overactivity in children
- PMID: 33305474
- PMCID: PMC7839517
- DOI: 10.1002/nau.24588
OnabotulinumtoxinA for the treatment of neurogenic detrusor overactivity in children
Abstract
Aims: This study evaluated whether one (or more) of three doses of onabotulinumtoxinA were safe and effective to treat neurogenic detrusor overactivity (NDO) in children.
Methods: This was a 48-week prospective, multicenter, randomized, double-blind study in children (aged 5-17 years) with NDO and urinary incontinence (UI) receiving one onabotulinumtoxinA treatment (50, 100, or 200 U; not to exceed 6 U/kg). Primary endpoint: change from baseline in daytime UI episodes. Secondary endpoints: change from baseline in urine volume at first morning catheterization, urodynamic measures, and positive response on the treatment benefit scale. Safety was also assessed.
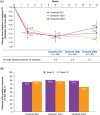
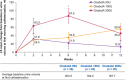
Results: There was a similar reduction in urinary incontinence from baseline to Week 6 for all doses (-1.3 episodes/day). Most patients reported positive responses on the treatment benefit scale (75.0%-80.5%). From baseline to Week 6, increases were observed in urine volume at first morning clean intermittent catheterization (50 U, 21.9 ml; 100 U, 34.9 ml; 200 U, 87.5 ml; p = 0.0055, 200 U vs. 50 U) and in maximum cystometric capacity (range 48.6-63.6 ml) and decreases in maximum detrusor pressure during the storage phase (50 U, -12.9; 100 U, -20.1; 200 U, -27.3 cmH2 O; p = 0.0157, 200 U vs. 50 U). The proportion of patients experiencing involuntary detrusor contractions dropped from baseline (50 U, 94.4%; 100 U, 88.1%; 200 U, 92.6%) to Week 6 (50 U, 61.8%; 100 U, 44.7%; 200 U, 46.4%). Safety was similar across doses; urinary tract infection was most frequent.
Conclusions: OnabotulinumtoxinA was well tolerated and effective for the treatment of NDO in children; 200 U showed greater efficacy in reducing bladder pressure and increasing bladder capacity.
Keywords: Type A; botulinum toxins; neurogenic; pediatrics; urinary bladder.
© 2020 The Authors. Neurourology and Urodynamics Published by Wiley Periodicals LLC.
Conflict of interest statement
Dr. Austin has served as a consultant/advisor to Allergan, an AbbVie company. Dr. Franco has served as a consultant/advisor to Allergan, an AbbVie company, and is chief science officer of FIAS Inc. (a software company) and chief science officer for Gogoband Inc. (a software and bedwetting device maker). Dr. Dobremez has served as a consultant for Allergan, an AbbVie company. Pawel Kroll has served as a study investigator for Allergan, an AbbVie company. Dr. Titanji, Dr. Geib, and Ms. Jenkins are employees of AbbVie, and may hold AbbVie stock. Dr. Hoebeke has served as a consultant for Allergan, an AbbVie company and Astellas.
Figures

Comment in
-
Pediatric Urology.J Urol. 2022 Jan;207(1):216-218. doi: 10.1097/JU.0000000000002267. Epub 2021 Oct 18. J Urol. 2022. PMID: 34661433 No abstract available.
References
-
- Rovner E, Dmochowski R, Chapple C, Thompson C, Lam W, Haag‐Molkenteller C. OnabotulinumtoxinA improves urodynamic outcomes in patients with neurogenic detrusor overactivity. Neurourol Urodyn. 2013;32:1109‐1115. - PubMed
-
- Guys JM, Hery G, Haddad M, Borrionne C. Neurogenic bladder in children: basic principles, new therapeutic trends. Scand J Surg. 2011;100:256‐263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

