Rehabilitation following rotator cuff repair: A multi-centre pilot & feasibility randomised controlled trial (RaCeR)
- PMID: 33305619
- PMCID: PMC8191146
- DOI: 10.1177/0269215520978859
Rehabilitation following rotator cuff repair: A multi-centre pilot & feasibility randomised controlled trial (RaCeR)
Abstract
Objective: To evaluate the feasibility of a multi-centre randomised controlled trial to compare the clinical and cost-effectiveness of early patient-directed rehabilitation versus standard rehabilitation following surgical repair of the rotator cuff of the shoulder.
Design: Two-arm, multi-centre pilot and feasibility randomised controlled trial.
Setting: Five National Health Service hospitals in England.
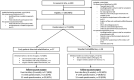
Participants: Adults (n = 73) with non-traumatic rotator cuff tears scheduled for repair were recruited and randomly allocated remotely prior to surgery.
Interventions: Early patient-directed rehabilitation (n = 37); advised to remove their sling as soon as able and move as symptoms allow. Standard rehabilitation (n = 36); sling immobilisation for four weeks.
Measures: (1) Randomisation of 20% or more eligible patients. (2) Difference in time out of sling of 40% or more between groups. (3) Follow-up greater than 70%.
Results: 73/185 (39%) potentially eligible patients were randomised. Twenty participants were withdrawn, 11 due to not receiving rotator cuff repair. The between-group difference in proportions of participants who exceeded the cut-off of 222.6 hours out of the sling was 50% (80% CI = 29%, 72%), with the early patient-directed rehabilitation group reporting greater time out of sling. 52/73 (71%) and 52/53 (98%) participants were followed-up at 12 weeks when withdrawals were included and excluded respectively. Eighteen full-thickness re-tears were reported (early patient-directed rehabilitation = 7, standard rehabilitation = 11). Five serious adverse events were reported.
Conclusion: A main randomised controlled trial is feasible but would require allocation of participants following surgery to counter the issue of withdrawal due to not receiving surgery.
Keywords: Rehabilitation interventions; physiotherapy; randomized controlled trial; shoulder pain.
Conflict of interest statement
Figures
Similar articles
-
Protocol for a multi-centre pilot and feasibility randomised controlled trial with a nested qualitative study: rehabilitation following rotator cuff repair (the RaCeR study).Trials. 2019 Jun 6;20(1):328. doi: 10.1186/s13063-019-3407-3. Trials. 2019. PMID: 31171031 Free PMC article.
-
Clinical and cost-effectiveness of individualised (early) patient-directed rehabilitation versus standard rehabilitation after surgical repair of the rotator cuff of the shoulder: protocol for a multicentre, randomised controlled trial with integrated Quintet Recruitment Intervention (RaCeR 2).BMJ Open. 2024 Apr 5;14(4):e081284. doi: 10.1136/bmjopen-2023-081284. BMJ Open. 2024. PMID: 38580365 Free PMC article.
-
Rehabilitation following rotator cuff repair: A nested qualitative study exploring the perceptions and experiences of participants in a randomised controlled trial.Clin Rehabil. 2021 Jun;35(6):911-919. doi: 10.1177/0269215520984025. Epub 2020 Dec 27. Clin Rehabil. 2021. PMID: 33356517 Free PMC article. Clinical Trial.
-
Surgery for rotator cuff tears.Cochrane Database Syst Rev. 2019 Dec 9;12(12):CD013502. doi: 10.1002/14651858.CD013502. Cochrane Database Syst Rev. 2019. PMID: 31813166 Free PMC article.
-
Patch augmentation surgery for rotator cuff repair: the PARCS mixed-methods feasibility study.Health Technol Assess. 2021 Feb;25(13):1-138. doi: 10.3310/hta25130. Health Technol Assess. 2021. PMID: 33646096 Free PMC article.
Cited by
-
Outcomes of Reverse Total Shoulder Arthroplasty Were Not Adversely Affected by the COVID-19 Pandemic.Semin Arthroplasty. 2023 Jun 21. doi: 10.1053/j.sart.2023.05.006. Online ahead of print. Semin Arthroplasty. 2023. PMID: 37362778 Free PMC article.
-
A Focus on Personalized Care in Light of New Diagnostic and Therapeutic Pathways of Shoulder and Elbow Disease.J Clin Med. 2025 Feb 20;14(5):1425. doi: 10.3390/jcm14051425. J Clin Med. 2025. PMID: 40094851 Free PMC article.
-
Use of a Spinal Needle Through the Deep Rotator Cuff Tissue to Treat Rotator Cuff Tears Under Direct Articular Vision.Arthrosc Tech. 2024 Mar 8;13(5):102960. doi: 10.1016/j.eats.2024.102960. eCollection 2024 May. Arthrosc Tech. 2024. PMID: 38835475 Free PMC article.
-
A Comparison Between Preoperative and Intraoperative Measurement and Classification of the Size of Rotator Cuff Tears.Rev Bras Ortop (Sao Paulo). 2022 Feb 9;58(2):356-360. doi: 10.1055/s-0041-1741445. eCollection 2023 Apr. Rev Bras Ortop (Sao Paulo). 2022. PMID: 37252312 Free PMC article.
-
Return to driving following rotator cuff repair: 23%, 70% and 99% at 1, 2 and 6 months.J Exp Orthop. 2025 Apr 16;12(2):e70201. doi: 10.1002/jeo2.70201. eCollection 2025 Apr. J Exp Orthop. 2025. PMID: 40242188 Free PMC article.
References
-
- Littlewood C, May S, Walters S. Epidemiology of rotator cuff tendinopathy: a systematic review. Shoulder Elb 2013; 5(4): 256–265.
-
- American Academy of Orthopaedic Surgeons. Management of rotator cuff injuries clinical practice guideline, https://www.aaos.org/globalassets/quality-and-practice-resources/rotator... (2019, accessed March 11 2019). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical