Coronavirus disease 2019 (COVID-19), human erythrocytes and the PKC-alpha/-beta inhibitor chelerythrine -possible therapeutic implication
- PMID: 33305655
- PMCID: PMC7781621
- DOI: 10.1080/15384101.2020.1859197
Coronavirus disease 2019 (COVID-19), human erythrocytes and the PKC-alpha/-beta inhibitor chelerythrine -possible therapeutic implication
Abstract
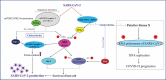
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes COVID-19. Until now, diverse drugs have been used for the treatment of COVID-19. These drugs are associated with severe side effects, e.g. induction of erythrocyte death, named eryptosis. This massively affects the oxygen (O2) supply of the organism. Therefore, three elementary aspects should be considered simultaneously: (1) a potential drug should directly attack the virus, (2) eliminate virus-infected host cells and (3) preserve erythrocyte survival and functionality. It is known that PKC-α inhibition enhances the vitality of human erythrocytes, while it dose-dependently activates the apoptosis machinery in nucleated cells. Thus, the use of chelerythrine as a specific PKC-alpha and -beta (PKC-α/-β) inhibitor should be a promising approach to treat people infected with SARS-CoV-2.
Keywords: RNA polymerases; SARS-CoV-2; chelerythrine; erythrocytes; mammalian target of rapamycin complex 1 & 2 (mTORC1/-2); protein kinase C-alpha (PKC-α).
Conflict of interest statement
The authors declare that no competing financial interests or otherwise exist.
Figures
Similar articles
-
The specific PKC-α inhibitor chelerythrine blunts costunolide-induced eryptosis.Apoptosis. 2020 Oct;25(9-10):674-685. doi: 10.1007/s10495-020-01620-6. Apoptosis. 2020. PMID: 32638182 Free PMC article.
-
Triggering of Eryptosis, the Suicidal Erythrocyte Death by Mammalian Target of Rapamycin (mTOR) inhibitor Temsirolimus.Cell Physiol Biochem. 2017;42(4):1575-1591. doi: 10.1159/000479398. Epub 2017 Jul 24. Cell Physiol Biochem. 2017. PMID: 28793293
-
Characterization of Suicidal Erythrocyte Death (Eryptosis) in Dogs.Cell Physiol Biochem. 2020 Jun 17;54(4):605-614. doi: 10.33594/000000243. Cell Physiol Biochem. 2020. PMID: 32543797
-
Therapeutic potential of chelerythrine as a multi-purpose adjuvant for the treatment of COVID-19.Cell Cycle. 2021 Nov;20(22):2321-2336. doi: 10.1080/15384101.2021.1982509. Epub 2021 Sep 29. Cell Cycle. 2021. PMID: 34585628 Free PMC article. Review.
-
mTOR inhibition in COVID-19: A commentary and review of efficacy in RNA viruses.J Med Virol. 2021 Apr;93(4):1843-1846. doi: 10.1002/jmv.26728. Epub 2020 Dec 17. J Med Virol. 2021. PMID: 33314219 Free PMC article. Review.
Cited by
-
Morphology and Function of Red Blood Cells in COVID-19 Patients: Current Overview 2023.Life (Basel). 2024 Apr 1;14(4):460. doi: 10.3390/life14040460. Life (Basel). 2024. PMID: 38672731 Free PMC article.
-
De novo production of protoberberine and benzophenanthridine alkaloids through metabolic engineering of yeast.Nat Commun. 2024 Oct 9;15(1):8759. doi: 10.1038/s41467-024-53045-3. Nat Commun. 2024. PMID: 39384562 Free PMC article.
-
COVID-19 induces proatherogenic alterations in moderate to severe non-comorbid patients: A single-center observational study.Blood Cells Mol Dis. 2021 Dec;92:102604. doi: 10.1016/j.bcmd.2021.102604. Epub 2021 Sep 9. Blood Cells Mol Dis. 2021. PMID: 34517295 Free PMC article.
-
Molecular Mechanisms and Pathophysiological Significance of Eryptosis.Int J Mol Sci. 2023 Mar 7;24(6):5079. doi: 10.3390/ijms24065079. Int J Mol Sci. 2023. PMID: 36982153 Free PMC article. Review.
-
Phenylethanoid glycosides as a possible COVID-19 protease inhibitor: a virtual screening approach.J Mol Model. 2021 Nov 3;27(11):341. doi: 10.1007/s00894-021-04963-2. J Mol Model. 2021. PMID: 34731296 Free PMC article.
References
-
- Klein U, Goossens T, Fischer M, et al. Somatic hypermutation in normal and transformed human B cells. Immunol Rev. 1998;162:261–280. - PubMed
-
- Li Z, Woo CJ, Iglesias-Ussel MD, et al. The generation of antibody diversity through somatic hypermutation and class switch recombination. Genes Dev. 2004;18:1–11. - PubMed
-
- Lee GS, Brandt VL, Roth DB.. B cell development leads off with a base hit: dU:dG mismatches in class switching and hypermutation. Mol Cell. 2004;16:505–508. - PubMed
-
- Steinhauer DA, Holland JJ. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
