Vitamin D supplementation for term breastfed infants to prevent vitamin D deficiency and improve bone health
- PMID: 33305822
- PMCID: PMC8812278
- DOI: 10.1002/14651858.CD013046.pub2
Vitamin D supplementation for term breastfed infants to prevent vitamin D deficiency and improve bone health
Abstract
Background: Vitamin D deficiency is common worldwide, contributing to nutritional rickets and osteomalacia which have a major impact on health, growth, and development of infants, children and adolescents. Vitamin D levels are low in breast milk and exclusively breastfed infants are at risk of vitamin D insufficiency or deficiency.
Objectives: To determine the effect of vitamin D supplementation given to infants, or lactating mothers, on vitamin D deficiency, bone density and growth in healthy term breastfed infants.
Search methods: We used the standard search strategy of Cochrane Neonatal to 29 May 2020 supplemented by searches of clinical trials databases, conference proceedings, and citations.
Selection criteria: Randomised controlled trials (RCTs) and quasi-RCTs in breastfeeding mother-infant pairs comparing vitamin D supplementation given to infants or lactating mothers compared to placebo or no intervention, or sunlight, or that compare vitamin D supplementation of infants to supplementation of mothers.
Data collection and analysis: Two review authors assessed trial eligibility and risk of bias and independently extracted data. We used the GRADE approach to assess the certainty of evidence.
Main results: We included 19 studies with 2837 mother-infant pairs assessing vitamin D given to infants (nine studies), to lactating mothers (eight studies), and to infants versus lactating mothers (six studies). No studies compared vitamin D given to infants versus periods of infant sun exposure. Vitamin D supplementation given to infants: vitamin D at 400 IU/day may increase 25-OH vitamin D levels (MD 22.63 nmol/L, 95% CI 17.05 to 28.21; participants = 334; studies = 6; low-certainty) and may reduce the incidence of vitamin D insufficiency (25-OH vitamin D < 50 nmol/L) (RR 0.57, 95% CI 0.41 to 0.80; participants = 274; studies = 4; low-certainty). However, there was insufficient evidence to determine if vitamin D given to the infant reduces the risk of vitamin D deficiency (25-OH vitamin D < 30 nmol/L) up till six months of age (RR 0.41, 95% CI 0.16 to 1.05; participants = 122; studies = 2), affects bone mineral content (BMC), or the incidence of biochemical or radiological rickets (all very-low certainty). We are uncertain about adverse effects including hypercalcaemia. There were no studies of higher doses of infant vitamin D (> 400 IU/day) compared to placebo. Vitamin D supplementation given to lactating mothers: vitamin D supplementation given to lactating mothers may increase infant 25-OH vitamin D levels (MD 24.60 nmol/L, 95% CI 21.59 to 27.60; participants = 597; studies = 7; low-certainty), may reduce the incidences of vitamin D insufficiency (RR 0.47, 95% CI 0.39 to 0.57; participants = 512; studies = 5; low-certainty), vitamin D deficiency (RR 0.15, 95% CI 0.09 to 0.24; participants = 512; studies = 5; low-certainty) and biochemical rickets (RR 0.06, 95% CI 0.01 to 0.44; participants = 229; studies = 2; low-certainty). The two studies that reported biochemical rickets used maternal dosages of oral D3 60,000 IU/day for 10 days and oral D3 60,000 IU postpartum and at 6, 10, and 14 weeks. However, infant BMC was not reported and there was insufficient evidence to determine if maternal supplementation has an effect on radiological rickets (RR 0.76, 95% CI 0.18 to 3.31; participants = 536; studies = 3; very low-certainty). All studies of maternal supplementation enrolled populations at high risk of vitamin D deficiency. We are uncertain of the effects of maternal supplementation on infant growth and adverse effects including hypercalcaemia. Vitamin D supplementation given to infants compared with supplementation given to lactating mothers: infant vitamin D supplementation compared to lactating mother supplementation may increase infant 25-OH vitamin D levels (MD 14.35 nmol/L, 95% CI 9.64 to 19.06; participants = 269; studies = 4; low-certainty). Infant vitamin D supplementation may reduce the incidence of vitamin D insufficiency (RR 0.61, 95% CI 0.40 to 0.94; participants = 334; studies = 4) and may reduce vitamin D deficiency (RR 0.35, 95% CI 0.17 to 0.72; participants = 334; studies = 4) but the evidence is very uncertain. Infant BMC and radiological rickets were not reported and there was insufficient evidence to determine if maternal supplementation has an effect on infant biochemical rickets. All studies enrolled patient populations at high risk of vitamin D deficiency. Studies compared an infant dose of vitamin D 400 IU/day with varying maternal vitamin D doses from 400 IU/day to > 4000 IU/day. We are uncertain about adverse effects including hypercalcaemia.
Authors' conclusions: For breastfed infants, vitamin D supplementation 400 IU/day for up to six months increases 25-OH vitamin D levels and reduces vitamin D insufficiency, but there was insufficient evidence to assess its effect on vitamin D deficiency and bone health. For higher-risk infants who are breastfeeding, maternal vitamin D supplementation reduces vitamin D insufficiency and vitamin D deficiency, but there was insufficient evidence to determine an effect on bone health. In populations at higher risk of vitamin D deficiency, vitamin D supplementation of infants led to greater increases in infant 25-OH vitamin D levels, reductions in vitamin D insufficiency and vitamin D deficiency compared to supplementation of lactating mothers. However, the evidence is very uncertain for markers of bone health. Maternal higher dose supplementation (≥ 4000 IU/day) produced similar infant 25-OH vitamin D levels as infant supplementation of 400 IU/day. The certainty of evidence was graded as low to very low for all outcomes.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MLT has no interest to declare.
SAA was an Advisory Board member for the Milk Processors Educational Program (MilkPep), and received consultancy as a scientific advisor. This relationship ended in December 2018.
DAO has no interest to declare.
Figures
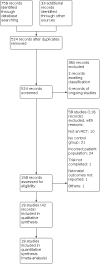
































































































Update of
References
References to studies included in this review
Ala‐Houhala 1985 {published data only}
Ala‐Houhala 1986 {published data only}
Alonso 2011 {published data only}
-
- Alonso A, Rodriguez J, Carvajal I, Prieto MA, Rodriguez RM, Perez AM, et al, Collaborative Group on Prophylaxis with Vitamin D in Asturias. Prophylactic vitamin D in healthy infants: assessing the need. Metabolism: Clinical and Experimental 2011;60(12):1719-25. [DOI: 10.1016/j.metabol.2011.04.011] [PMID: ] - DOI - PubMed
Chandy 2016 {published data only}
-
- Chandy DD, Kare J, Singh SN, Agarwal A, Das V, Singh U, et al. Effect of vitamin D supplementation, directly or via breast milk for term infants, on serum 25 hydroxyvitamin D and related biochemistry, and propensity to infection: a randomised placebo-controlled trial. British Journal of Nutrition 2016;116(1):52-8. [DOI: 10.1017/S0007114516001756] [PMID: ] - DOI - PubMed
Greer 1981 {published data only}
-
- Greer FR, Searcy JE, Levin RS, Steichen JJ, Steichen-Asche PS, Tsang RC. Bone mineral content and serum 25-hydroxyvitamin D concentrations in breast-fed infants with and without supplemental vitamin D: one-year follow-up. Journal of Pediatrics 1982;100(6):919-22. [DOI: 10.1016/s0022-3476(82)80514-3] [PMID: ] - DOI - PubMed
Greer 1989 {published data only}
Hollis 2015 {published data only}
-
- Reading R. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Child: Care, Health & Development 2016;42:148. [DOI: 10.1111/cch.12310] - DOI
-
- Roth DE. Maternal postpartum high-dose vitamin D3 supplementation (6400 IU/day) or conventional infant vitamin D3 supplementation (400 IU/day) lead to similar vitamin D status of healthy exclusively/fully breastfeeding infants by 7 months of age. Evidence-Based Medicine 2016;21(2):75. [DOI: 10.1136/ebmed-2015-110354.] - DOI - PubMed
Madar 2009 {published data only}
Moodley 2015 {published data only}
Naik 2017 {published data only}
-
- Naik P, Faridi MM, Batra P, Madhu SV. Oral supplementation of parturient mothers with vitamin D and its effect on 25OHD status of exclusively breastfed infants at 6 months of age: a double-blind randomized placebo controlled trial. Breastfeeding Medicine 2017;12(10):621-8. [DOI: 10.1089/bfm.2016.0164] [PMID: ] - DOI - PubMed
Niramitmahapanya 2017 {published data only}
-
- Niramitmahapanya S, Kaoiean S, Sangtawesin V, Bordeerat NK, Deerochanawong C. Correlation of 25-hydroxyvitamin D levels in serum vs breastmilk in vitamin D-supplementation breastfeeding women during lactation: randomized double blinded control trial. In: Endocrine Reviews. Conference: 99th Annual Meeting of the Endocrine Society, ENDO 2017. United States. Vol. 38. 2017. - PubMed
-
- Niramitmahapanya S, Kaoiean S, Sangtawesin V, Patpanaprapan A, Bordeerat NK. Maternal vitamin D3 supplementation during lactation ameliorate vitamin D status of breast-fed infants: randomized controlled trial. In: Endocrine Reviews. Conference: 97th Annual Meeting and Expo of the Endocrine Society, ENDO 2015. United States. Vol. 36. 2015.
-
- NCT02297568. Vitamin D supplementation during lactation [Randomized control trial of vitamin D supplementation during lactation on vitamin D in maternal milk]. clinicaltrials.gov/ct2/show/NCT02297568 (first received 21 November 2014).
-
- Niramitmahapanya S, Kaoiean S, Sangtawesin V, Patanaprapan A, Bordeerat NK, Deerochanawong C. Correlation of 25-hdroxyvitamin D levels in serum vs. breastmilk in vitamin D-supplementation breastfeeding women during lactation: randomized double blinded control trial. Journal of the Medical Association of Thailand 2017;100(Suppl 1):S165-71. [PMID: ] - PubMed
-
- Niramitmahapanya S, Kaoiean S, Sangtawesin V, Patpanaprapan A, Bordeerat NK, Deerochanawong C. Effect on vitamin D status of breastfeeding infants after vitamin D3 supplementation during breastfeeding lactation: a double-blind randomized controlled trial. Annals of Clinical Endorinology and Metabolism 2017;1:6-14. [DOI: 10.29328/journal.hcem.1001002] - DOI
Ponnapakkam 2010 {published data only}
-
- Bradford EM, Gensure R, Ponnapakkam T. Vitamin D supplementation in the breastfed infants: preliminary results of a prospective trial. Journal of Investigative Medicine 2010;58:448.
-
- Ponnapakkam T, Bradford E, Gensure R. Vitamin D supplementation in breastfed infants: results of a prospective trial in the southern United States. Journal of Bone and Mineral Research 2010;25:S232.
Roth 2016 {published data only}
-
- NCT03537443. Bone and muscle health in kids (BONUSKids) [Effect of maternal vitamin D supplementation during pregnancy on offspring bone mass, body composition and muscle strength in early childhood: follow-up of a randomized controlled trial cohort.]. clinicaltrialsgov/show/NCT03537443 (first received 25 May 2018).
-
- Roth DE, Gernand AD, Morris SK, Pezzack B, Islam MM, Dimitris MC, et al. Maternal vitamin D supplementation during pregnancy and lactation to promote infant growth in Dhaka, Bangladesh (MDIG trial): study protocol for a randomized controlled trial. Trials 2015;16:300. [DOI: 10.1186/s13063-015-0825-8] [PMID: ] - DOI - PMC - PubMed
Rothberg 1982 {published data only}
Rueter 2019 {published data only}
-
- Kristina K, Jones AP, Siafarikas A, Lim E-M, Prescott SL, Palmer DJ. In "high-risk" infants with sufficient vitamin D status at birth, infant vitamin D supplementation had no effect on allergy outcomes: a randomized controlled trial. Nutrients 2020;12(6):1747. [DOI: 10.3390/nu12061747] [PMID: ] - DOI - PMC - PubMed
-
- Rueter K, Jones AP, Siafarikas A, Lim EM, Clarke MW, Noakes PS, et al. Does early oral vitamin d supplementation and UV-light exposure have an impact on allergic disease outcome in infancy? Internal Medicine Journal 2019;49(Suppl 4):35.
Thiele 2017 {published data only}
-
- Anderson CM, Thiele DK, Ralph JL, Perley D, Ohm JE. Vitamin D supplementation and DNA methylation patterns during pregnancy and lactation in mothers and infants. Federation of American Societies for Experimental Biology Journal 2016;30(S!):1028.3. [DOI: 10.1096/fasebj.30.1_supplement.1028.3] - DOI
Trivedi 2020 {published data only}
-
- Trivedi M, Faridi MM, Aggarwal A, Madhu SV, Malhotra RK. Oral vitamin D supplementation to mothers during lactation - effect of 25(OH)D concentration on exclusively breastfed infants at 6 months of age: a randomized double-blind placebo-controlled trial. Breastfeeding Medicine 2020;15:237-45. [DOI: 10.1089/bfm.2019.0102] [PMID: ] - DOI - PubMed
Wagner 2006 {published data only}
Wheeler 2016 {published data only}
-
- Wheeler BJ, Taylor BJ, Herbison P, Haszard JJ, Mikhail A, Jones S, et al. Effect of high dose monthly maternal cholecalciferol supplementation during breastfeeding on infant and maternal vitamin d status at 5 months post-partum: a randomized controlled trial. International Journal of Pediatric Endocrinology 2017;2017 (Suppl 1)(15):35.
-
- Wheeler BJ, Taylor BJ, Herbison P, Haszard JJ, Mikhail A, Jones S, et al. High-dose monthly maternal cholecalciferol supplementation during breastfeeding affects maternal and infant vitamin D status at 5 months postpartum: a randomized controlled trial. Journal of Nutrition 2016;146(10):1999-2006. [DOI: 10.3945/jn.116.236679] [PMID: ] - DOI - PubMed
References to studies excluded from this review
Abdel‐Hady 2019 {published data only}
ACTRN12613000732785 {published data only}
-
- ACTRN12613000732785. The efficacy of vitamin D supplementation in infants on bone mineral content: a double blind randomized controlled trial [Vitamin D infant bone mineral content study]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000732785 (first received 20 June 2013).
ACTRN12618001174279 {published data only}
-
- ACTRN12618001174279. Vitamin D supplementation to prevent acute respiratory infections among Indigenous children in the Northern Territory: a randomised controlled trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375278 (first received 9 July 2018).
Al‐Beltagi 2020 {published data only}
Backstrom 1999 {published data only}
-
- Backstrom MC, Maki R, Kuusela AL, Sievanen H, Koivisto AM, Koskinen M, et al. The long-term effect of early mineral, vitamin D, and breast milk intake on bone mineral status in 9- to 11-year-old children born prematurely. Journal of Pediatric Gastroenterology and Nutrition 1999;29(5):575-82. [DOI: 10.1097/00005176-199911000-00019] [PMID: ] - DOI - PubMed
Bagnoli 2013 {published data only}
-
- Bagnoli F, Casucci M, Toti S, Cecchi S, Iurato C, Coriolani G, et al. Is vitamin D supplementation necessary in healthy full-term breastfed infants? A follow-up study of bone mineralization in healthy full-term infants with and without supplemental vitamin D. Minerva Pediatrica 2013;65(3):253-60. [PMID: ] - PubMed
Baird 2016 {published data only}
Basile 2006 {published data only}
Bugrul 2013 {published data only}
-
- Bugrul F, Devecioglu E, Ozden T, Gokcay G, Omer B. Effect of maternal and infant vitamin D supplementation on vitamin D levels of breastfed infants. Turkish Journal of Pediatrics 2013;55(2):158-63. [PMID: ] - PubMed
Challa 2005 {published data only}
Chan 1982 {published data only}
Chawes 2016 {published data only}
-
- Brustad N, Eliasen A, Stokholm J, Bonnelykke K, Bisgaard H, Chawes BL. High-dose vitamin D supplementation during pregnancy and asthma at age 6: a randomized controlled trial. Allergy: European Journal of Allergy and Clinical Immunology 2019;74(Suppl 106):60. [DOI: 10.1001/jama.2019.0052] [PMID: ] - DOI - PMC - PubMed
-
- Kofod Vinding R, Stokholm J, Sevelsted A, Chawes BL, Bonnelykke K, Barman M, et al. Fish oil supplementation in pregnancy increases gestational age, size for gestational age, and birth weight in infants: a randomized controlled trial. Journal of Nutrition 2019;149(4):628-34. [DOI: 10.1093/jn/nxy204] [PMID: ] - DOI - PubMed
Cooper 2016 {published data only}
-
- Bishop NJ, Kennedy S, Papageorghiou AT, Fraser R, Gandhi SV, D'Angelo S, et al. Maternal gestational vitamin D supplementation and offspring bone mass: a multicentre randomised, double-blind, placebo-controlled trial (MAVIDOS). In: Journal of Bone and Mineral Research. Conference: 2015 Annual Meeting of the American Society for Bone and Mineral Research, ASBMR 2015. United States. Vol. 30. 2015.
-
- Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, et al, MAVIDOS Study Group. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes and Endocrinology 2016;4:393-402. [DOI: 10.1016/S2213-8587(16)00044-9] [PMID: ] - DOI - PMC - PubMed
Czech‐Kowalska 2013 {published data only}
-
- Czech-Kowalska J, Latka-Grot J, Bulsiewicz D, Jaworski M, Pludowski P, Chazan B, et al. Influence of vitamin D supplementation on vitamin D status and bone mass during lactation - double blinded randomized control trial. In: Annals of Nutrition & Metabolism. Vol. 63. 2013:796.
-
- Czech-Kowalska J, Latka-Grot J, Bulsiewicz D, Jaworski M, Pludowski P, Wygledowska G, et al. Impact of vitamin D supplementation during lactation on vitamin D status and body composition of mother-infant pairs: a MAVID randomized controlled trial. PLOS One 2014;9(9):e107708. [DOI: 10.1371/journal.pone.0107708] [PMID: ] - DOI - PMC - PubMed
-
- Czech-Kowalska J, Latka-Grot J, Bulsiewicz D, Jaworski M, Wygledowska G, Chazan B, et al. Influence of vitamin D supplementation in lactating mothers on their and offspring's vitamin D status and bone mass - double blinded randomized control trial - MAVID study. Journal of Perinatal Medicine 2013;41(S1):594.
Dawudo 2019 {published data only}
-
- Dawodu A, Salameh K, Reedy A. Very low human milk vitamin D in breastfeeding mothers in an environment with abundant sunlight. In: FASEB Journal. Conference: Experimental Biology 2016, EB. San Diego, CA United States. Vol. 30. 2016.
-
- Dawodu A, Salameh KM, Al-Janahi NS, Bener A, Elkum N. The effect of high-dose postpartum maternal vitamin D supplementation alone compared with maternal plus infant vitamin D supplementation in breastfeeding infants in a high-risk population. A randomized controlled trial. Nutrients 2019;11(7):1632. [DOI: 10.3390/nu11071632] [PMID: ] - DOI - PMC - PubMed
-
- Salameh K, Dawodu AH. Randomized controlled study of effectiveness and safety of high dose vitamine D supplementation on breast milk v D limited sun. In: Journal of Pediatric Gastroenterology and Nutrition. Conference: 51st Annual Meeting European Society for Paediatric Gastroenterology, Hepatology and Nutrition, ESPGHAN 2018. Switzerland. Vol. 66. 2018:1097.
Delvin 2005 {published data only}
-
- Delvin EE, Salle BL, Claris O, Putet G, Hascoet JM, Desnoulez L, et al. Oral vitamin A, E and D supplementation of pre-term newborns either breast-fed or formula-fed: a 3-month longitudinal study. Journal of Pediatric Gastroenterology and Nutrition 2005;40(1):43-7. [DOI: 10.1097/00005176-200501000-00008] [PMID: ] - DOI - PubMed
Diogenes 2013 {published data only}
-
- Diogenes ME, Bezerra FF, Rezende EP, Donangelo CM. Calcium plus vitamin D supplementation during the third trimester of pregnancy in adolescents accustomed to low calcium diets does not affect infant bone mass at early lactation in a randomized controlled trial. Journal of Nutrition 2015;145(7):1515-23. [DOI: 10.3945/jn.114.208140] [PMID: ] - DOI - PubMed
-
- Diogenes ME, Bezerra FF, Rezende EP, Taveira MF, Normando P, Pinhal I, et al. Infant and maternal bone status are associated in lactating adolescents when calcium intake is low but not following calcium plus vitamin D supplementation. Federation of American Societies for Experimental Biology Journal 2013;27:850.5. [DOI: 10.1096/fasebj.27.1_supplement.850.5] - DOI
-
- Diogenes ME, Bezerra FF, Rezende EP, Taveira MF, Pinhal I, Donangelo CM. Effect of calcium plus vitamin D supplementation during pregnancy in Brazilian adolescent mothers: a randomized, placebo-controlled trial. American Journal of Clinical Nutrition 2013;98(1):82-91. [DOI: 10.3945/ajcn.112.056275] [PMID: ] - DOI - PubMed
-
- Diogenes ME, Bezerra FF, Rezende EP, Taveira MF, Pinhal I, Donangelo CM. Maternal vitamin D status of adolescent mothers at mid-pregnancy influence bone mineral content of their newborns. Federation of American Societies for Experimental Biology Journal 2011;25:603.19. [DOI: 10.1096/fasebj.25.1_supplement.603.19] - DOI
Francis 2018 {published data only}
-
- Francis J, Unger S, Bando N, Vance A, Gibbins S, Kiss A, et al, GTA-DoMINO Feeding Group. Postdischarge feeding of very-low-birth-weight infants: adherence to nutrition guidelines. Journal of Pediatric Gastroenterology and Nutrition 2018;67(3):401-8. [DOI: 10.1097/MPG.0000000000002041] [PMID: ] - DOI - PubMed
Galdo 2018 {published data only}
-
- Galdo AM, De Aguileta AL, Mercader PT, Estela AC, Marcos MIM, Murua JK, et al. Effect of supplementation with vitamin D on acute bronchitis prevention during the first year of life. In: European Respiratory Journal. Conference: European Respiratory Society International Congress, ERS 2018. France. Vol. 52. 2018.
Gallo 2013a {published data only}
-
- Gallo S, Rodd C, Vanstone C, Agellon S, L'Abbe M, Khamessan A, et al. Lumbar spine bone mineral density is enhanced in breast fed infants receiving 800 or 1200 IU of vitamin D daily from 4 to 20 weeks of age. In: Journal of Bone and Mineral Research. Conference: 2011 Annual Meeting of the American Society for Bone and Mineral Research, ASBMR 2011. San Diego, CA United States. Vol. 26. 2011.
-
- Gallo S, Comeau K, Vanstone C, Agellon S, Sharma A, Jones G, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA 2013;309:1785-92. [DOI: 10.1001/jama.2013.3404] [PMID: ] - PubMed
-
- Gallo S, Hazell T, Vanstone CA, Agellon S, Jones G, L'Abbe M, et al. Vitamin D supplementation in breastfed infants from Montreal, Canada: 25-hydroxyvitamin D and bone health effects from a follow-up study at 3 years of age. Osteoporosis International 2016;27(8):2459-66. [DOI: 10.1007/s00198-016-3549-z] [PMID: ] - DOI - PubMed
-
- Gallo S, Vanstone CA, Comeau K, Agellon S, Rodd C, Sharma A, et al. Vitamin D dose response study in canadian infants: 25-Hydroxy-vitamin D status during a 20 week intervention. In: Federation of American Socieities for Experimental Biology Journal. Vol. 24. 2010. [DOI: 10.1096/fasebj.24.1_supplement.537.11] - DOI
Gallo 2013b {published data only}
-
- Gallo S, Phan A, Vanstone CA, Rodd C, Weiler HA. The change in plasma 25-hydroxyvitamin D did not differ between breast-fed infants that received a daily supplement of ergocalciferol or cholecalciferol for 3 months. Journal of Nutrition 2013;143(2):148-53. [DOI: 10.3945/jn.112.167858] [PMID: ] - DOI - PMC - PubMed
Grant 2014 {published data only}
Gupta 2018 {published data only}
-
- Gupta T, Sharma H, Bajpai J, Kachhawa G, Kulshreshtha V, Khadgawat R, et al. A randomized double blind controlled trial to investigate the effects of vitamin D supplementation on maternal and new-born baby's vitamin D status in Asian-Indian subjects. Indian Journal of Endocrinology and Metabolism 2018;22(7 Suppl 1):S89.
Hibbs 2018 {published data only}
Ho 1985 {published data only}
Hollis 2004 {published data only}
-
- Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. American Journal of Clinical Nutrition 2004;80(6 Suppl):1752s-8s. [DOI: 10.1093/ajcn/80.6.1752S] [PMID: ] - DOI - PubMed
Huynh 2015 {published data only}
-
- Huynh J, Lu T, Liew D, Doery J, Tudball R, Jona M, et al. The Vine study: vitamin D in newborns: an RCT comparing daily and bolus supplementation. In: Journal of Paediatrics and Child Health. Conference: 19th Annual Meeting of the Perinatal Society of Australia and New Zealand, PSANZ 2015. Melbourne, VIC Australia. Vol. 51; S1. 2015:30-1.
-
- ACTRN12613001234707. A randomised controlled trial evaluating the efficacy and safety of single bolus (50,000 international units) versus daily (400 international units) dosing of vitamin D in healthy breastfed infants of vitamin D deficient mothers [A randomised controlled trial comparing daily (400 international units) and single bolus dosing (50,000 international units) of vitamin D in healthy breastfed infants of vitamin D deficient mother]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613001234707 (first received 9 October 2013).
-
- Huynh J, Liew D, Rodda C. Neonatal vitamin D supplementation. Journal of Paediatrics and Child Health 2017;53(7):725-6. [DOI: 10.1111/jpc.13582] [PMID: ] - PubMed
Ketha 2018 {published data only}
-
- Ketha H, Thacher TD, Oberhelman SS, Fischer PR, Singh RJ, Kumar R. Comparison of the effect of daily versus bolus dose maternal vitamin D3 supplementation on the 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 ratio. Bone 2018;110:321-5. [DOI: 10.1016/j.bone.2018.02.024] [PMID: ] - DOI - PMC - PubMed
Kishore 2019 {published data only}
-
- Kishore SS, Gadiraju M. Study of daily vitamin D supplementation in preterm infants: a randomized trial. Journal of Pediatric Gastroenterology and Nutrition 2019;68:1167.
Kolodziejczyk 2017 {published data only}
Kuryaninova 2017 {published data only}
-
- Kuryaninova V, Dolbnya S, Kasyanova A, Yagupova A, Zakharova I, Klimov L, et al. B-defensin level changes over time in children under three years of age receiving cholecalciferol. Journal of Pediatric Gastroenterology and Nutrition 2017;64:988.
Lara‐Corrales 2013 {published data only}
-
- Lara-Corrales I, Gomez GR, De Los Rios CP, Pope E. Vitamin D in patients with atopic dermatitis: a randomized, double-blinded, placebo-controlled study preliminary analysis. Pediatric Dermatology 2013;30:638.
Litonjua 2014 {published data only}
-
- Lu M, Carey VJ, O'Connor GT, Bacharier LB, Zeiger RS, Hollis B, et al. Prenatal and neonatal vitamin D sufficiency are associated with reduced risk of childhood asthma/recurrent wheeze. In: American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference, ATS 2018. United States. Vol. 197. 2018.
-
- Mirzakhani H, O'Connor GT, Bacharier L, Zeiger RS, Schatz MX, Weiss S, et al. Asthma control status during pregnancy and its relationship with vitamin D level: an observation from the vitamin D antenatal asthma reduction trial (VDAART). In: American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference, ATS 2017. United States. Vol. 195. 2017.
-
- Hornsby E, Pfeffer PE, Laranjo N, Cruikshank W, Tuzova M, Litonjua AA, et al. Vitamin D supplementation during pregnancy: effect on the neonatal immune system in a randomized controlled trial. Journal of Allergy and Clinical Immunology 2018;141(1):269-78. [DOI: 10.1016/j.jaci.2017.02.039] [PMID: ] - DOI - PubMed
-
- Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O'Connor GT, et al. Effect of prenatal supplementation with Vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA 2016;315(4):362-70. [DOI: 10.1001/jama.2015.18589] [PMID: ] - DOI - PMC - PubMed
-
- Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemporary Clinical Trials 2014;38(1):37-50. [DOI: 10.1016/j.cct.2014.02.006] [PMID: ] - DOI - PMC - PubMed
March 2015 {published data only}
-
- Barr SI, Lyon MR, Whiting SJ, Weiler HA, Green TJ. Maternal vitamin D₃ supplementation at 50 μg/d protects against low serum 25-hydroxyvitamin D in infants at 8 wk of age: a randomized controlled trial of 3 doses of vitamin D beginning in gestation and continued in lactation. American Journal of Clinical Nutrition 2015;102(2):402-10. [DOI: 10.3945/ajcn.114.106385] [PMID: 27802998 ] - DOI - PubMed
-
- Chen NN, March K, Innis SM, Shand A, Von Dadelszen P, Lyon M, et al. The effect of vitamin D supplementation during pregnancy and lactation on maternal & infant 25-hydroxyvitamin D (25OHD) concentration. Federation of American Societies for Experimental Biology Journal 2013;27:lb259. [DOI: 10.1096/fasebj.27.1_supplement.lb259] - DOI
-
- March KM, Chen NN, Karakochuk CD, Shand AW, Innis SM, Von Dadelszen P, Barr SI, Lyon MR, Whiting SJ, Weiler HA, Green TJ. Maternal vitamin D3 supplementation at 50 mg/d protects against low serum 25-hydroxyvitamin D in infants at 8 wk of age: A randomized controlled trial of 3 doses of vitamin D beginning in gestation and continued in lactation. American Journal of Clinical Nutrition 2015;102:402-10; erratum (American Journal of Clinical Nutrition (2015) 102 (402-410).. - PubMed
Mirghafourvand 2015 {published data only}
-
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Mansouri A, Najafi M, Khodabande F. The effect of vitamin D and calcium plus vitamin D on sleep quality in pregnant women with leg cramps: a controlled randomized clinical trial. Journal of Isfahan Medical School 2015;32(320):2444-53. [CENTRAL: CN-01070330]
-
- Mohammad-Alizadeh-Charandabi S, Mirghafourvand M, Mansouri A, Najafi M, Khodabande F. The effect of vitamin D and calcium plus vitamin D during pregnancy on pregnancy and birth outcomes: a randomized controlled trial. Journal of Caring Sciences 2015;4(1):35-44. [DOI: 10.5681/jcs.2015.004] [PMID: ] - DOI - PMC - PubMed
Morris 2017 {published data only}
-
- Morris SK, Pell LG, Rahman MZ, Dimitris MC, Mahmud A, Islam MM, et al. Maternal vitamin D supplementation during pregnancy and lactation to prevent acute respiratory infections in infancy in Dhaka, Bangladesh (MDARI trial): protocol for a prospective cohort study nested within a randomized controlled trial. BMC Pregnancy and Childbirth 2016;16(1):309. [DOI: 10.1186/s12884-016-1103-9] [PMID: ] - DOI - PMC - PubMed
-
- Morris SK, Pell LG, Rahman MZ, Gubbay J, Pullenayegum E, Ahmed T, et al. Maternal vitamin d supplementation during pregnancy and lactation to prevent acute respiratory infections in infancy in Dhaka, Bangladesh (MDARI trial): a prospective cohort study nested within a randomized controlled trial during pregnancy and lactation to prevent respiratory infections in infancy in Bangladesh (MDARI trial). In: American Journal of Tropical Medicine and Hygiene. Vol. 97. 2017:387.
Nausheen 2018 {published data only}
-
- Nausheen S, Soofi S. Assessment of dose effectiveness of vitamin D supplementation during pregnancy. A dose comparison clinical trial. BJOG: An International Journal of Obstetrics and Gynaecology 2018;125:15.
NCT02713009 {published data only}
-
- NCT02713009. Impact of maternal body weight on vitamin D status during pregnancy (MO-VITD) [Investigation of the impact of maternal body weight on vitamin D status during pregnancy: a randomised supplementation study]. clinicaltrials.gov/ct2/show/NCT02713009 (first received 18 March 2016).
Norizoe 2014 {published data only}
O'Callaghan 2018 {published data only}
-
- O'Callaghan KM, Hennessy A, Hull GL, Healy K, Ritz C, Kenny LC, et al. Estimation of the maternal vitamin D intake that maintains circulating 25-hydroxy vitamin D in late gestation at a concentration sufficient to keep umbilical cord sera >= 25-30 nmol/L: a dose-response, double-blind, randomized placebo-controlled trial in pregnant women at northern latitude. American Journal of Clinical Nutrition 2018;108(1):77-91. [DOI: 10.1093/ajcn/nqy064] [PMID: ] - DOI - PMC - PubMed
Oberhelman 2013 {published data only}
-
- Ketha H, Thacher TD, Oberhelman SS, Fischer PR, Singh RJ, Kumar R. Comparison of the effect of daily versus bolus dose maternal vitamin D3 supplementation on the 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 ratio. Bone 2018;110:321-5. [DOI: 10.1016/j.bone.2018.02.024] [PMID: ] - DOI - PMC - PubMed
-
- Oberhelman SS, Meekins M, Fischer P, Lee B, Gardner B, Singh R, et al. Maternal vitamin D supplementation to optimize the vitamin D status of breastfed infants. Federation of American Societies for Experimental Biology Journal 2012;26(S1):44.5. [DOI: 10.1096/fasebj.26.1_supplement.44.5] - DOI
Onal 2010 {published data only}
Perumal 2017 {published data only}
-
- Perumal N, Mahmud A, Baqui A, Roth D. Prenatal vitamin D supplementation and infant vitamin D status in Bangladesh. In: FASEB Journal. Conference: Experimental Biology 2014, EB. San Diego, CA United States. Vol. 28. 2014.
-
- Dimitris MC, Perumal N, Craig-Barnes HA, Leadley M, Mahmud AA, Baqui AH, et al. Effect of weekly high-dose vitamin D3 supplementation on serum cholecalciferol concentrations in pregnant women. Journal of Steroid Biochemistry and Molecular Biology 2016;158:76-81. [DOI: 10.1016/j.jsbmb.2016.01.007] [PMID: ] - DOI - PubMed
-
- Perumal N, Al Mahmud A, Baqui A, Raqib R, Roth D. Effect of high-dose vitamin D supplementation on blood pressure during the third trimester of pregnancy: a randomized controlled trial in Bangladesh. American Journal of Epidemiology 2012;176(1):80.
Rasmussen 2015 {published data only}
-
- Rasmussen GB, Mosekilde L, Sikjaer T, Vestergaard P, Heickendorff L, Uldbjerg N, et al. Effects of high dose vitamin D supplementation on bone metabolism in pregnant women with hypovitaminosis D - a randomized controlled trial. In: Journal of Bone and Mineral Research. Conference: 2015 Annual Meeting of the American Society for Bone and Mineral Research, ASBMR 2015. United States. Vol. 30. 2015.
Roberfroid 2012 {published data only}
-
- Roberfroid D, Huybregts L, Lanou H, Ouedraogo L, Henry MC, Meda N, et al, MISAME study group. Impact of prenatal multiple micronutrients on survival and growth during infancy: a randomized controlled trial. American Journal of Clinical Nutrition 2012;95(4):916-24. [DOI: 10.3945/ajcn.111.029033] [PMID: ] - DOI - PubMed
Roberts 1981 {published data only}
Rosendahl 2017 {published data only}
-
- Enlund-Cerullo M, Koljonen L, Holmlund-Suila E, Hauta-Alus H, Rosendahl J, Valkama S, et al. Genetic variation of the vitamin D binding protein affects vitamin D status and response to supplementation in infants. Journal of Clinical Endocrinology and Metabolism 2019;104(11):5483-98. [DOI: 10.1210/jc.2019-00630] [PMID: ] - DOI - PubMed
-
- Hauta-Alus HH, Kajantie E, Holmlund-Suila EM, Rosendahl J, Valkama SM, Enlund-Cerullo M, et al. High pregnancy, cord blood, and infant vitamin D concentrations may predict slower infant growth. Journal of Clinical Endocrinology & Metabolism 2019;104(2):397-407. [DOI: 10.1210/jc.2018-00602] [PMID: ] - DOI - PubMed
-
- Helve O, Viljakainen H, Holmlund-Suila E, Rosendahl J, Hauta-alus H, Enlund-Cerullo M, et al. Towards evidence-based vitamin D supplementation in infants: Vitamin D Intervention in Infants (VIDI) - study design and methods of a randomised controlled double-blinded intervention study. BMC Pediatrics 2017;17(1):91. [DOI: 10.1186/s12887-017-0845-5] [PMID: ] - DOI - PMC - PubMed
-
- Kampe A, Enlund-Cerullo M, Valkama S, Holmlund-Suila E, Rosendahl J, Hauta-Alus H, et al. Genetic variation in GC and CYP2R1 affects 25-hydroxyvitamin D concentration and skeletal parameters: a genome-wide association study in 24-month-old Finnish children. PLOS Genetics 2019;15(12):e1008530. [DOI: 10.1371/journal.pgen.1008530] [PMID: ] - DOI - PMC - PubMed
-
- NCT04302987. Vitamin D intervention in infants - 6 years follow-up (VIDI2). clinicaltrialsgov/show/NCT04302987 (first received 10 March 2020).
Rostami 2018 {published data only}
-
- Rostami M, Tehrani FR, Simbar M, Bidhendi Yarandi R, Minooee S, Hollis BW, et al. Effectiveness of prenatal vitamin D deficiency screening and treatment program: a stratified randomized field trial. Journal of Clinical Endocrinology & Metabolism 2018;103(8):2936-48. [DOI: 10.1210/jc.2018-00109] [PMID: ] - DOI - PubMed
Saadi 2009 {published data only}
-
- Saadi HF, Dawodu A, Afandi B, Zayed R, Benedict S, Nagelkerke N, et al. Effect of combined maternal and infant vitamin D supplementation on vitamin D status of exclusively breastfed infants. Maternal & Child Nutrition 2009;5(1):25-32. [DOI: 10.1111/j.1740-8709.2008.00145.x] [PMID: ] - DOI - PMC - PubMed
Salas 2018 {published data only}
-
- Salas AA, Woodfin T, Phillips V, Peralta-Carcelen M, Carlo WA, Ambalavanan N. Dose-desponse effects of early vitamin D supplementation on neurodevelopmental and respiratory outcomes of extremely preterm infants at 2 years of age: a randomized trial. Neonatology 2018;113(3):256-62. [DOI: 10.1159/000484399] [PMID: ] - DOI - PMC - PubMed
Savino 2011 {published data only}
Shakiba 2010 {published data only}
-
- Shakiba M, Sadr S, Nefei Z, Mozaffari-Khosravi H, Lotfi MH, Bemanian MH. Combination of bolus dose vitamin D with routine vaccination in infants: a randomised trial. Singapore Medical Journal 2010;51(5):440-5. [PMID: ] - PubMed
Siafarikas 2011 {published data only}
-
- Siafarikas A, Piazena H, Feister U, Bulsara MK, Meffert H, Hesse V. Randomised controlled trial analysing supplementation with 250 versus 500 units of vitamin D3, sun exposure and surrounding factors in breastfed infants. Archives of Disease in Childhood 2011;96(1):91-5. [DOI: 10.1136/adc.2009.178301] [PMID: ] - DOI - PubMed
Terashita 2017 {published data only}
Tomimoto 2018 {published data only}
-
- Tomimoto K. A study of vitamin D supplementation in breast fed infant with vitamin D deficiency [ビタミンD欠乏状態にある母乳栄養児における 適切なビタミンD補充の検討 ]. Journal of the Japan Pediatric Society 2018;122(11):1683-91.
Wagner 2013 {published data only}
-
- Wagner CL, Howard CR. Maternal vitamin D supplementation (vitD-S) during lactation: results of a two-site RCT. Breastfeeding Medicine 2013;8:S-2.
-
- Wagner CL, McNeil R, Hamilton SA, Winkler J, Rodriguez Cook C, Warner G, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. American Journal of Obstetrics and Gynecology 2013;208(2):137.e1-.e13. [DOI: 10.1016/j.ajog.2012.10.888] [PMID: ] - DOI - PMC - PubMed
Zamora 1999 {published data only}
Ziegler 2014 {published data only}
References to studies awaiting assessment
Kim 2010 {published data only}
Wagner 2018 {published data only}
-
- Wagner C, Newton DA, Baatz JE. Maternal vitamin D (VitD) status affects hepatitis B vaccine (HBV) response in breastfeeding infants. In: Breastfeeding Medicine.Conference: 19th International Society for Research in Human Milk and Lactation conference, ISRHML Japan. Vol. 13. 2018:A-12. [CENTRAL: CN-01669515]
References to ongoing studies
ACTRN12614000334606 {published data only}
-
- ACTRN12614000334606. A placebo-controlled, randomised trial of vitamin D supplementation for infants in their first year of life, to prevent the development of food allergy by age 12 months. The VITALITY Trial [Can vitamin D supplementation in infants prevent food allergy in the first year of life? The VITALITY Trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366006&isRe... (first received 20 March 2014).
-
- Allen KJ, Panjari M, Koplin JJ, Ponsonby AL, Vuillermin P, Gurrin LC, et al. VITALITY trial: protocol for a randomised controlled trial to establish the role of postnatal vitamin D supplementation in infant immune health. BMJ Open 2015;5(12):e009377. [DOI: 10.1136/bmjopen-2015-009377] - DOI - PMC - PubMed
ACTRN12615000642583 {published data only}
-
- ACTRN12615000642583. Infants of vitamin D deficient mothers - trial comparing pentavite and vitamin D3 supplement - effect on vitamin D level at 6 weeks [Infants of vitamin D deficient mothers - trial comparing 2 supplement options with vitamin D levels repeated at 6 weeks]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362437 (first received 24 April 2012).
ACTRN12618001992291 {unpublished data only}
-
- ACTRN12618001992291. The Vaccination Infant Supplementation (VISS) Study - assessing the effect of vitamin D and probiotic supplementation around vaccination on infant's temperature and sleep pattern [Randomised placebo-controlled trial investigating the effect of 8 weeks supplementation with probiotics and vitamin D around routine childhood immunisation on infant's ear temperature, growth, and sleeping pattern in 4-24 month-old infants.]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=376526&isReview... (first received 5 December 2018).
ChiCTR1800020179 {unpublished data only}
-
- ChiCTR1800020179. Study for vitamins and fatty acids status of breast milk and effects of related supplementation during lactation on the health of mothers and infants: a randomized clinical trial [Study for vitamins and fatty acids status of breast milk and effects of related supplementation during lactation on the breast milk composition and the health of mothers and infants: a randomized clinical trial]. www.chictr.org.cn/showprojen.aspx?proj=33888 (first received 19 December 2018).
Additional references
Adams 2010
Andiran 2002
Armas 2004
Butte 2001
-
- Butte NF, Lopez-Alarcon MG, Garza C. Nutrient adequacy of exclusive breastfeeding for the term infant during the first six months of life. In: Expert Consultation on the Optimal Duration of Exclusive Breastfeeding. Geneva: World Health Organization, 2001.
Clarke 2008
Crabtree 2014
-
- Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. Journal of Clinical Densitometry 2014;17:225-42. - PubMed
Creo 2017
Crocker 2011
Dawodu 2003
-
- Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. Journal of Pediatrics 2003;142(2):169-73. [DOI: 10.1067/mpd.2003.63] [PMID: ] - DOI - PubMed
EFSA 2016
-
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), Scientific opinion on dietary reference values for vitamin D. Dietary reference values for vitamin D. European Food Safety Authority Journal 2016;14(10):4547. [DOI: 10.2903/j.efsa.2016.4547] - DOI
Elder 2014
Fraser 1967
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version (accessed 26 June 2018). Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Haggerty 2010
Haugen 2016
Health Canada 2012
-
- Health Canada. Vitamin D and calcium: updated dietary reference intakes. www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/vi... (accessed 25 July 2017).
Heinig 2003
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2017
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Holick 1980
Holick 1981
Holick 1992
Hollis 1981
IOM 2011
Kasalová 2015
Koo 2013
Lerch 2007
Lovell 2016
-
- Lovell AL, Wall CR, Grant CC. Do maternal dietary vitamin D intake and sunlight exposure affect the vitamin D status of exclusively breastfed infants? Nutrition & Dietetics 2016;73(5):420-6. [DOI: 10.1111/1747-0080.12254] - DOI
Major 2013
Matsuoka 1992
Merewood 2012
Misra 2008
-
- Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M, Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 2008;122(2):398-417. [DOI: 10.1542/peds.2007-1894] [PMID: ] - DOI - PubMed
Munns 2016
NHS 2017
-
- National Health Service, United Kingdom. Sun safety for children. www.nhs.uk/conditions/pregnancy-and-baby/pages/safety-in-the-sun.aspx# (accessed 25 January 2017).
NICE 2014
-
- National Institute for Health and Care Excellence. Vitamin D: increasing supplement use in at-risk groups (Public Health Guideline PH56). www.nice.org.uk/guidance/ph56 (accessed 1 February 2017).
Oliveri 2015
Palacios 2019a
Palacios 2019b
Perrine 2010
Pettifor 2004
Pezzuti 2017
Pharange 2015
-
- Pharande P, Pammi M, Collins CT, Zhou SJ, Abrams SA. Vitamin D supplementation for prevention of vitamin D deficiency in preterm and low birth weight infants. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD011529. [DOI: 10.1002/14651858.CD011529] - DOI
Pludowski 2013
-
- Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality - a review of recent evidence. Autoimunity Reviews 2013;12(10):976-89. [DOI: 10.1016/j.autrev.2013.02.004] [PMID: ] - DOI - PubMed
Prentice 2013
RevMan 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Roth 2018
-
- Roth DE, Abrams SA, Aloia J, Bergeron G, Bourassa MW, Brown KH, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Annals of the New York Academy of Sciences 2018;1430(1):44-79. [DOI: 10.1111/nyas.13968] [PMID: ] - DOI - PMC - PubMed
Salameh 2016
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shore 2013a
Shore 2013b
Taylor 2010
Umaretiya 2017
US Dietary Guidelines Advisory Committee 2020
-
- Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. www.dietaryguidelines.gov/2020-advisory-committee-report (accessed 20 August 2020).
Við Streym 2016
Wagner 2008
-
- Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122(5):1142-52. [DOI: 10.1542/peds.2008-1862] [PMID: ] - DOI - PubMed
Wharton 2003
WHO 2003
-
- World Health Organization, UNICEF. Global strategy for infant and young child feeding. whqlibdoc.who.int/publications/2003/9241562218.pdf (accessed 24 January 2017).
Winzenberg 2010
Winzenberg 2013a
Winzenberg 2013b
-
- Winzenberg TM, Shaw KA, Van der Mei IAF, Jones G. Vitamin D supplementation in infancy for improving bone density. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD010658. [DOI: 10.1002/14651858.CD010658] - DOI
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

