Trypanocidal drugs for late-stage, symptomatic Chagas disease (Trypanosoma cruzi infection)
- PMID: 33305846
- PMCID: PMC8103997
- DOI: 10.1002/14651858.CD004102.pub3
Trypanocidal drugs for late-stage, symptomatic Chagas disease (Trypanosoma cruzi infection)
Abstract
Background: People with Chagas disease may develop progressive and lethal heart conditions. Drugs to eliminate the parasite Trypanosoma cruzi (T cruzi) currently carry limited therapeutic value and are used in the early stages of the disease. Extending the use of these drugs to treat chronic chagasic cardiomyopathy (CCC) has also been proposed.
Objectives: To assess the benefits and harms of nitrofurans and trypanocidal drugs for treating late-stage, symptomatic Chagas disease and CCC in terms of blood parasite reduction or clearance, mortality, adverse effects, and quality of life.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and LILACS databases on 12 November 2019. We also searched two clinical trials registers, ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), on 3 December 2019.
Selection criteria: We included randomised controlled trials (RCTs) assessing trypanocidal drugs versus placebo or no treatment for late-stage, symptomatic Chagas disease and CCC.
Data collection and analysis: We conducted the reporting of the review according the standard Cochrane methods. Two review authors independently retrieved articles, performed data extraction, and assessed risk of bias. Any disagreements were resolved by a third review author. We contacted study authors for additional information.
Main results: We included two studies in this review update. One RCT randomly assigned 26 participants to benznidazole 5 mg/kg/day; 27 participants to nifurtimox 5 mg/kg/day; and 24 participants to placebo for 30 days. The second RCT, newly included in this update, randomised 1431 participants to benznidazole 300 mg/day for 40 to 80 days and 1423 participants to placebo. We also identified one ongoing study. Benznidazole compared to placebo At five-year follow-up, low quality of the evidence suggests that there may be a benefit of benznidazole when compared to placebo for clearance or reduction of antibody titres (risk ratio (RR) 1.25, 95% confidence interval (CI) 1.14 to 1.37; 1 trial; 1896 participants). We are uncertain about the effects of benznidazole for the clearance of parasitaemia demonstrated by negative xenodiagnosis, blood culture, and/or molecular assays due to very limited evidence. Low quality of the evidence suggests that when compared to placebo, benznidazole may make little to no difference in the risk of heart failure (RR 0.89, 95% CI 0.69 to 1.14; 1 trial; 2854 participants) and ventricular tachycardia (RR 0.80, 95% CI 0.51 to 1.26; 1 trial; 2854 participants). We found moderate quality of the evidence that adverse events increase with benznidazole when compared to placebo (RR 2.52, 95% CI 2.09 to 3.03; 1 trial; 2854 participants). Adverse effects were observed in 23.9% of patients in the benznidazole group compared to 9.5% in the placebo group. The most frequent adverse effects were: cutaneous rash, gastrointestinal symptoms, and peripheral polyneuropathy. No data were available for the outcomes of pathological demonstration of tissue parasites and quality of life. Nifurtimox compared to placebo Data were only available for this comparison for the outcome clearance or reduction of antibody titres, and we are uncertain about the effect due to very limited evidence. Regarding adverse events, one RCT mentioned in a general manner that nifurtimox caused intense adverse events, without any quantification.
Authors' conclusions: There is insufficient evidence to support the efficacy of the trypanocidal drugs benznidazole and nifurtimox for late-stage, symptomatic Chagas disease and CCC.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
There is no conflict of interest in this work.
MV: none known.
PAR: none known.
MMG: none known.
AGG: none known.
Figures
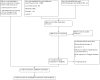






Update of
-
Trypanocidal drugs for late stage, symptomatic Chagas disease (Trypanosoma cruzi infection).Cochrane Database Syst Rev. 2005 Oct 19;(4):CD004102. doi: 10.1002/14651858.CD004102.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2020 Dec 11;12:CD004102. doi: 10.1002/14651858.CD004102.pub3. PMID: 16235350 Updated.
References
References to studies included in this review
Coura 1997 {published data only}
-
- Coura JR, De Abreu L, Percy H, Willcox F, Petana W. A controlled comparative study using benznidazole, nifurtimox and placebo in chronic Chagas disease patients, in a field area with interrupted transmission: I. preliminary evaluation [Estudo comparativo controlado com emprego de benznidazole, nifurtimox e placebo, na forma crônica da doença de Chagas, em uma área de campo com transmissäo interrompida: I. avaliaçäo preliminar]. Revista da Sociedade Brasileira de Medicina Tropical 1997;30:139-44. [DOI: 10.1590/s0037-86821997000200009] - DOI - PubMed
References to studies excluded from this review
Aguiar 2012 {published data only}
-
- Aguiar C, Batista A, Pavan T, Almeida E, Guariento M, Wanderleyn J, et al. Serological profiles and evaluation of parasitaemia by PCR and blood culture in individuals chronically infected by Trypanosoma cruzi treated with benzonidazole. Tropical Medicine & International Health 2012;17(3):368-73. [DOI: 10.1111/j.1365-3156.2011.02936.x] - DOI - PubMed
Alvarez 2012 {published data only}
Alvarez 2016 {published data only}
-
- Álvarez M, Hernández Y, Bertocchi G, Fernández M, Lococo B, Ramírez J, et al. New scheme of intermittent benznidazole administration in patients chronically infected with Trypanosoma cruzi: a pilot short-term follow-up study with adult patients. Antimicrobial Agents and Chemotherapy 2016;60:833–7. [DOI: 10.1128/AAC.00745-15] - DOI - PMC - PubMed
Bertocchi 2013 {published data only}
-
- Bertocchi GL, Vigliano C, Lococo B, Petti M, Viotti R. Clinical characteristics and outcome of 107 adult patients with chronic Chagas disease and parasitological cure criteria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2013;107:372–6. [DOI: 10.1093/trstmh/trt029] - DOI - PubMed
Bestetti 2011 {published data only}
Braga 2000 {published data only}
Bravo 2011 {published data only}
-
- Bravo I, Parra F, Nello C, Rodríguez-Bonfante C, Useche F, Bonfante-Cabarcas R. Prevalence of Trypanosoma cruzi antibodies and inflammatory markers in uncompensated heart failure. Revista da Sociedade Brasileira de Medicina Tropical 2011;44(6):691-6. [DOI: 10.1590/s0037-86822011000600008] - DOI - PubMed
Cancado 2002 {published data only}
-
- Cancado J. Long term evaluation of etiological treatment of Chagas disease with benznidazole. Revista do Instituto de Medicina Tropical de Sao Paulo 2002;44:29-37. [DOI: 10.1590/S0036-46652002000100006 ] - PubMed
EQUITY 2019 {published data only}
-
- Villar J, Herrera V, Pérez J, Váquiro E, Castellanos Y, Vásquez S, et al. Nifurtimox versus benznidazole or placebo for asymptomatic Trypanosoma cruzi infection (Equivalence of Usual Interventions for Trypanosomiasis - EQUITY): study protocol for a randomised controlled trial. Trials 2019;20(1):1-10. [DOI: 10.1186/s13063-019-3423-3] - DOI - PMC - PubMed
Fabbro 2000 {published data only}
-
- Fabbro de Suasnábar D, Arias E, Streiger M, Placenza M, Ingraramo M, Del Barco M, et al. Evolutive behavior towards cardiomyopathy of treated (nifurtimox or benznidazole) and untreated Chagasic patients. Revista do Instituto de Medicina Tropical de Sao Paulo 2000;42:99-109. [DOI: 10.1590/s0036-46652000000200007] - DOI - PubMed
Lauria‐Pires 2000 {published data only}
-
- Lauria-Pires L, Braga M, Vexenat A, Nitz N, Simoes-Barbosa A, Tinoco D, et al. Progressive chronic Chagas heart disease ten years after treatment with anti-Trypanosoma cruzi nitroderivatives. American Journal of Tropical Medicine and Hygiene 2000;63(3-4):111-8. [DOI: 10.4269/ajtmh.2000.63.111] - DOI - PubMed
Machado‐de‐Assis 2012 {published data only}
-
- Machado-de-Assis G, Silva A, Do Bem V, Bahia M, Martins-Filho O, Dias J, et al. Posttherapeutic cure criteria in Chagas' disease: conventional serology followed by supplementary serological, parasitological, and molecular tests. Clinical and Vaccine Immunology 2012;19(8):1283-91. [DOI: 10.1128/CVI.00274-12] - DOI - PMC - PubMed
Moreira 2013 {published data only}
-
- Moreira O, Ramírez J, Velázquez E, Melo M, Lima-Ferreira C, Guhl F, et al. Towards the establishment of a consensus real-time qPCR to monitor Trypanosoma cruzi parasitaemia in patients with chronic Chagas disease cardiomyopathy: a substudy from the BENEFIT trial. Acta Tropica 2013;125(1):23-31. [DOI: 10.1016/j.actatropica.2012.08.020] - DOI - PubMed
Moreno 2010 {published data only}
Murcia 2010 {published data only}
-
- Murcia L, Carrilero B, Muñoz M, Iborra M, Segovia M. Usefulness of PCR for monitoring benznidazole response in patients with chronic Chagas’ disease: a prospective study in a non-disease-endemic country. Journal of Antimicrobial Chemotherapy 2010;65(8):1759-64. [DOI: 10.1093/jac/dkq201] - DOI - PubMed
Perez‐Ayala 2010 {published data only}
SaMiTrop 2018 {published data only}
TRAENA 2015 {published data only}
-
- Riarte A. TRENA: Treatment with benznidazole in adult patients with chronic Chagas' disease. A phase 3 randomised clinical trial (RCT) double blinded [TRAENA: TRAtamiento con benznidazol EN pacientes Adultos con enfermedad de Chagas crónica. Un ensayo clínico aleatorizado (ECA) a doble ciego en Fase 3]. Informativo. Plataforma de investigación clínica en enfermedad de Chagas 2015;4:6-7.
Viotti 1994 {published data only}
Viotti 2006 {published and unpublished data}
-
- Viotti R, Viglian C, Lococo B, Bertochi G, Petti M, Alvarez M, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a non-randomised trial. Annals of Internal Medicine 2006;144:724-34. [DOI: 10.7326/0003-4819-144-10-200605160-00006] - DOI - PubMed
References to ongoing studies
NCT03191162 {unpublished data only}
-
- NCT03191162. Evaluation of different benznidazole regimens for the treatment of chronic Chagas disease (MULTIBENZ). clinicaltrials.gov/ct2/show/NCT03191162 (first received 19 June 2017).
Additional references
Andrade 2011
Barretto 1990
-
- Barretto A, Azul L, Mady C, Ianni B, De Brito V, Belloti G, et al. Indeterminate form of Chagas' disease. A polymorphic disease [Forma indeterminada da doenca de chagas: Uma doenca polimorfica]. Arquivos Brasileiros de Cardiologia 1990;55:347-53. [PMID: ] - PubMed
Brener 1975
Briceno 2016
Cecere 1999
-
- Cecere M, Castañera M, Canale D, Chuit R, Gürtler R. Trypanosoma cruzi infection in Triatoma infestans and other triatomines: long-term effects of a control program in rural northwestern Argentina. Revista Panamericana de Salud Publica [Pan American Journal of Public Health] 1999;5:392-9. [DOI: 10.1590/s1020-49891999000500003] - DOI - PubMed
Chagas 1909
-
- Chagas C. New human trypanosomia. Studies on the morphology and evolutionary cycle of the Schizotrypanum cruzi n.gen., N.sp., ethiological adjunct of a new morbid entity of man [Nova tripanozomiaze humana. Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n.gen., n.sp., ajente etiolojico de nova entidade morbida do homem]. Memorias do Instituto Oswaldo Cruz 1909;1:159-218. [DOI: 10.1590/S0074-02761909000200008] - DOI
Cochrane Heart Group
-
- Cochrane Heart Group. Preferred method for handling continuous variables. www.heart.cochrane.org/Files/Handling%20continuous%20variables.pdf (accessed June 2005).
Contijo 1999
Coura 2009
de Andrade 1996
Dias 2009
Follman 1992
Fumadó 2014
Gobbi 2014
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 10 December 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Grant 1989
Harry 2000
Higgins 2002
Higgins 2003
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
Hozo 2005
Imai 2015
Kraus 2009
Kubata 2002
Laucella 2009
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lopez‐Monteon 2019
-
- López-Monteon A, Dumonteil E, Ramos-Ligonio A. More than a hundred years in the search for an accurate diagnosis for Chagas disease: current panorama and expectations. In: Rodriguez-Morales AJ, editors(s). Current Topics in Neglected Tropical Diseases. IntechOpen, 2019:4-14. [DOI: 10.5772/intechopen.86567] - DOI
Mady 2008
Monteón 1995
-
- Monteón V, Guzman-Bracho C, Floriani-Verdugo J, Ramos-Echavaria A, Velasco-Castrejón O, Reyes-López PA. Serological diagnosis of Chagas disease: self-sufficiency and interlaboratory concordance [Diagnóstico serológico de la enfermedad de Chagas: autosuficiencia y concordancia interlaboratorios]. Salud Publica de Mexico 1995;37:232-5. [PMID: ] - PubMed
Monteón‐Padilla 1999
-
- Monteón-Padilla V, Hernández-Becerril N, Guzmán-Bracho C, Rosales-Encina J, Reyes-López PA. American Trypanosomiasis (Chagas' disease) and blood banking in Mexico City: seroprevalence and its potential transfusional transmission risk. Archives of Medical Research 1999;30:393-8. [DOI: 10.1016/S0188-4409(99)00062-4] - DOI - PubMed
Moretti 1998
-
- Moretti E, Cervetta L, Basso B, Castro I, Santamarina N. Chronic Chagas' disease: effects of treatment on the levels of antibodies to crude and partially purified Trypanosoma cruzi antigens [Enfermedad de Chagas crónica: efectos del tratamiento en los niveles de anticuerpos hacia antígenos crudos y semipurificados del Trypanosoma cruzi]. Boletin Chileno de Parasitologia 1998;53:3-9. [PMID: ] - PubMed
Nickerson 1989
Oliveira‐Filho 1989
-
- Oliveira-Filho A. Cost-effectiveness analysis in Chagas' disease vector's control intervention. Memorias do Instituto Oswaldo Cruz 1989;84 (Suppl IV):409-17. [DOI: 10.1590/S0074-02761989000800074] - DOI
Perman 2017
-
- Perman G. Cluster clinical trials [Ensayos clínicos por conglomerados]. EVIDENCIA Actualizacion en la Practica Ambulatoria 2017;20(1):22-5.
Pinto 1995
-
- Pinto Dias JC. Natural history of Chagas disease [História natural da doenca de Chagas]. Arquivos Brasileiros de Cardiologia 1995;65:359-65. - PubMed
Ramos 2012
Ramsey 2014
Rassi 2007
-
- Rassi A, Luquetti A, Rassi A Jr, Rassi G, Rassi S, Da Silva IG, et al. Specific treatment for Trypanosoma cruzi: lack of efficacy of allopurinol in the human chronic phase of Chagas disease. American Journal of Tropical Medicine and Hygiene 2007;76(1):58-61. [PMID: ] - PubMed
Rassi 2010
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 2016
Rodriguez‐Coura 2002
Rojas 1999
Sales 2017
Salomao 2016
Sbaraglini 2016
Schijman 2011
-
- Schijman A, Bisio M, Orellana L, Sued M, Duffy T, Mejia A, et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLOS Neglected Tropical Diseases 2011;5(1):e931. [DOI: 10.1371/journal.pntd.0000931] - DOI - PMC - PubMed
Schmunis 1994
-
- Schmunis G. American trypanosomiasis as a public health problem. In: Chagas' Disease and the Nervous System. Washington, DC: Pan American Health Organization, World Health Organization, 1994:3-29.
Schmunis 1999
Schmuñis 2013
Schofield 1999
Schofield 2006
Schunemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations.. The GRADE Working Group. 2013. [Available from guidelinedevelopment. org/handbook.]
Segura 1994
Sosa 1998
Sterne 2000
Sterne 2001
Stoppani 1999
-
- Stoppani A. The chemotherapy of Chagas disease [Quimioterapia de la enfermedad de Chagas]. Medicina 1999;59(Suppl 2):147-65. [PMID: ] - PubMed
Tanowitz 1992
Tarleton 2014
Urbina 1999
Urbina 2009
Urbina 2015
Valerio‐Sallent 2012
-
- Valerio-Sallent L, Roure S, Basile L, Ballesteros L, Sabrià M, Rodrigo C, Grupo Metropolitano de estudio del Chagas. A clinical and epidemiological study of the Trypanosoma cruzi infected population in the north metropolitan area of Barcelona [Un estudio clínico-epidemiológico de la población infectada por Trypanosoma cruzi en la zona metropolitana norte de Barcelona]. Revista Clinica Espanola 2012;212(7):329-36. [DOI: 10.1016/j.rce.2012.03.017] - DOI - PubMed
Villar 2002
Villar 2014
WHO 1991
-
- World Health Organization. Control of Chagas' disease. Report of a WHO Expert Committee. WHO Technical Report Series No. 811. Geneva: World Health Organization, 1991. - PubMed
WHO 2015
-
- WHO/Department of control of neglected tropical diseases. Investing to overcome the global impact of neglected tropical diseases: Third WHO report on neglected tropical diseases 2015. Vol. 3. World Health Organization, 2015.
WHO 2017
-
- WHO/Department of Control of Neglected Tropical Diseases. Fourth WHO report on neglected tropical diseases. Vol. 1. Geneva: World Health Organization, 2017.
Yun 2009
-
- Yun O, Lima M, Ellman T, Chambi W, Castillo S, Flevaud L, et al. Feasibility, drug safety, and effectiveness of etiological treatment programs for Chagas disease in Honduras, Guatemala, and Bolivia: 10-year experience of Medecins Sans Frontières. PLOS Neglected Tropical Diseases 2009;3(7):e488. [DOI: 10.1371/journal.pntd.0000488] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

