Developing an advanced gut on chip model enabling the study of epithelial cell/fibroblast interactions
- PMID: 33306083
- PMCID: PMC9930731
- DOI: 10.1039/d0lc00672f
Developing an advanced gut on chip model enabling the study of epithelial cell/fibroblast interactions
Erratum in
-
Correction: Developing an advanced gut on chip model enabling the study of epithelial cell/fibroblast interactions.Lab Chip. 2023 Mar 14;23(6):1713. doi: 10.1039/d3lc90020g. Lab Chip. 2023. PMID: 36852524 Free PMC article.
Abstract
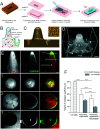
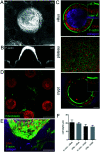
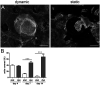
Organoids are widely used as a model system to study gut pathophysiology; however, they fail to fully reproduce the complex, multi-component structure of the intestinal wall. We present here a new gut on chip model that allows the co-culture of primary epithelial and stromal cells. The device has the topography and dimensions of the mouse gut and is based on a 3D collagen I scaffold. The scaffold is coated with a thin layer of laminin to mimic the basement membrane. To maintain the scaffold structure while preserving its cytocompatibility, the collagen scaffold was rigidified by threose-based post-polymerization treatment. This treatment being cytocompatible enabled the incorporation of primary intestinal fibroblasts inside the scaffold, reproducing the gut stromal compartment. We observed that mouse organoids, when deposited into crypts, opened up and epithelialized the scaffold, generating a polarized epithelial monolayer. Proper segregation of dividing and differentiated cells along the crypt-villus axis was achieved under these conditions. Finally, we show that the application of fluid shear stress allows the long-term culture of this intestinal epithelium. Our device represents a new biomimetic tool that captures key features of the gut complexity and could be used to study gut pathophysiology.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

