Effect of a Social Norm Email Feedback Program on the Unnecessary Prescription of Nimodipine in Ambulatory Care of Older Adults: A Randomized Clinical Trial
- PMID: 33306114
- PMCID: PMC7733153
- DOI: 10.1001/jamanetworkopen.2020.27082
Effect of a Social Norm Email Feedback Program on the Unnecessary Prescription of Nimodipine in Ambulatory Care of Older Adults: A Randomized Clinical Trial
Abstract
Importance: Nimodipine is a highly prescribed drug for the treatment of cognitive impairment and dementia in Argentina. There is little evidence to support the use of nimodipine for cognitive impairment and dementia.
Objective: To test the effectiveness of a behavioral intervention based on social norm feedback to reduce prescription of nimodipine for cognitive impairment in Argentina.
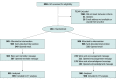
Design, setting, and participants: This pragmatic parallel-group randomized clinical trial included 2 arms with a 1:1 allocation ratio. General practitioner physicians in the national health care system for older adults in Argentina (INSSJP-PAMI) with history of high nimodipine prescription rate were enrolled. The study was conducted from May 2019 to October 2019, and data were analyzed from November 2019 to February 2020.
Interventions: The treatment group received 2 emails with evidence-based information about nimodipine plus the individual's level of nimodipine prescription compared with their peers. The control group received 2 emails with general information about the risks of overprescription in older adults.
Main outcomes and measures: The primary outcome was the cumulative number of nimodipine prescriptions per 1000 prescriptions of all drugs made by the targeted physicians during the 6 months of the study. Secondary outcomes included annual monetary savings attributable to the intervention and physicians' qualitative perceptions of the acceptability of the procedure.
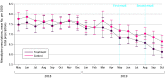
Results: Of 1811 physicians enrolled, 906 physicians (354 [39.1%] women; mean [SD] age, 57.10 [10.73] years) were randomized to treatment and 905 participants (331 [36.6%] women; mean [SD] age, 56.49 [10.47] years) to the control group. Physicians in the treatment group wrote a mean of 93.25 (95% CI, 89.27 to 97.24) prescriptions of nimodipine, compared with 98.99 (95% CI, 95.00 to 102.98) prescriptions among practitioners in the control group during the half-year of the intervention (mean difference, -5.73 [95% CI, -11.38 to -0.10] prescriptions; P = .046), which meant a 5.79% reduction. Regression analysis revealed a significant association of the group condition with number of prescriptions per 1000 total prescriptions when controlling for baseline prescriptions (B = -0.312 [95% CI, -0.465 to -0.160]; P < .001). The observed difference corresponds to a 4.48% reduction in nimodipine prescriptions per 1000 prescriptions of all drugs made by physicians in the treated group compared with the control group. Physicians who effectively opened the email in the treatment group (427 physicians [47.1%]) prescribed the drug 11.3% less compared with the control group (426 physicians) (mean difference, -10.78 [95% CI, -18.53 to -3.03] prescriptions; P = .006). Expenditures were 7.18% lower in the treatment group, resulting in an estimated annual net cost benefit of US $234 893.35 (95% CI, $225 565.35 to $237 112.30).
Conclusions and relevance: In this randomized clinical trial, the social norm email feedback program showed an effect on curbing the nonrecommended prescription of nimodipine. It was highly cost-effective and well accepted by participants.
Trial registration: ISRCTN.org identifier: ISRCTN17823729.