Taccalonolide Microtubule Stabilizers
- PMID: 33306174
- PMCID: PMC7800047
- DOI: 10.1007/978-3-030-52966-6_3
Taccalonolide Microtubule Stabilizers
Abstract
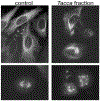
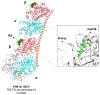
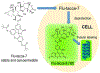
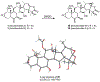
Microtubule stabilizers are a mainstay in the treatment of many solid cancers and continue to find utility in combination therapy with molecularly targeted anticancer agents and immunotherapeutics. However, innate and acquired resistance to microtubule stabilizers can limit their clinical efficacy. The taccalonolides are a unique class of microtubule stabilizers isolated from plants of Tacca that circumvent clinically relevant mechanisms of drug resistance. Although initial reports suggested that the microtubule-stabilizing activity of the taccalonolides was independent of direct tubulin binding, additional studies have identified that potent C-22, C-23 epoxidized taccalonolides covalently bind the Aspartate 226 residue of β-tubulin and that this interaction is critical for their microtubule-stabilizing activity. The taccalonolides have distinct properties as compared to other microtubule stabilizers with regard to their biochemical effects on tubulin structure and dynamics that promote distinct cellular phenotypes. Some taccalonolides have demonstrated in vivo antitumor efficacy in drug-resistant tumor models with exquisite potency and long-lasting antitumor efficacy as a result of their irreversible target engagement. The recent identification of a site on the taccalonolide scaffold that is amenable to modification has provided evidence of the specificity of the taccalonolide-tubulin interaction. This also affords an opportunity to further optimize the targeted delivery of the taccalonolides to further improve their anticancer efficacy and potential for clinical development.
Keywords: Antitumor natural product; Microtubule stabilizer; Microtubule-targeted agent; Taccalonolide.
Figures

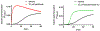


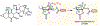
References
-
- Scheuer PJ, Swanholm CE, Madamba LA, Hudgins WR, Constituents of Tacca leontopetaloides. Lloydia (1962) 26:133
-
- Chen Z-L, Wang B-D, Chen M-Q (1987) Steroidal bitter principles from Tacca Plantaginea; structures of taccalonolide A and B. Tetrahedron Lett 28:1673
-
- Chen Z, Wang B, Shen J, (1988) Taccalonolide C and D, two pentacyclic steroids of Tacca plantaginea. Phytochem 27:2999
-
- Shen J, Chen Z, Gao Y (1996) The pentacyclic steroidal constituents of Tacca plantaginea: taccalonolide E and F. Chin J Chem 9:92
-
- Chen ZL, Shen JH Gao YS, Wichtl M (1997) Five taccalonolides from Tacca plantaginea. Planta Med 63:40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

