Treatment with compounded fluticasone suspension improves the clinical, endoscopic, and histologic features of eosinophilic esophagitis
- PMID: 33306783
- PMCID: PMC8275977
- DOI: 10.1093/dote/doaa120
Treatment with compounded fluticasone suspension improves the clinical, endoscopic, and histologic features of eosinophilic esophagitis
Abstract
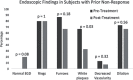
No approved medication exists for the treatment of eosinophilic esophagitis (EoE) in the United States, which forces patients to utilize off-label drugs and/or create their own formulations. We assessed the efficacy of a standardized compounded fluticasone suspension. To do this, we performed a retrospective cohort study identifying all EoE patients treated with compounded fluticasone. Compounded fluticasone was prescribed during routine clinical care and dispensed by a specialty compounding pharmacy. Clinical data were extracted from medical records. Outcomes (symptomatic, endoscopic, and histologic) were assessed after the initial and last compounded fluticasone treatment in our system. There were 27 included patients (mean age 34.2; 67% male; 96% white) treated for a mean length of 5.4 ± 4.4 months. The majority (89%) previously utilized dietary elimination or topical corticosteroids, and many (75%) had primary non-response or secondary loss of response to these treatments. After starting compounded fluticasone, symptoms and endoscopic findings improved [dysphagia (89 vs. 56%, P = 0.005), food impaction (59 vs. 4%, P = 0.003), heartburn (26 vs. 4%, P = 0.01), chest pain (26 vs. 8%, P = 0.05), white plaques (63 vs. 32%; P = 0.005), furrows (81 vs. 60%; P = 0.06), and edema (15 vs. 4%; P = 0.16)]. The median of the peak eosinophil counts decreased from 52 to 37 eos/hpf (P = 0.10) and 35% of patients achieved <15 eos/hpf. In conclusion, compounded fluticasone provided a significant improvement in symptoms and endoscopic findings, with more than a third achieving histologic response in a treatment refractory EoE population. Compounded fluticasone should be considered as an EoE management option.
Keywords: clinical pharmacy; eosinophilic esophagitis; fluticasone; outcomes research.
© The Author(s) 2020. Published by Oxford University Press on behalf of International Society for Diseases of the Esophagus. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Straumann A, Katzka D A. Diagnosis and treatment of eosinophilic esophagitis. Gastroenterology 2018; 154: 346–59. - PubMed
-
- Furuta G T, Liacouras C A, Collins M H et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133: 1342–63. - PubMed
-
- Landres R T, Kuster G G, Strum W B. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology 1978; 74: 1298–301. - PubMed
-
- Attwood S E, Smyrk T C, Demeester T R, Jones J B. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38: 109–16. - PubMed
-
- Straumann A, Spichtin H P, Bernoulli R, Loosli J, Vögtlin J. Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings. Schweiz Med Wochenschr 1994; 124: 1419–29. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical