Tafenoquine: a toxicity overview
- PMID: 33306921
- PMCID: PMC10591488
- DOI: 10.1080/14740338.2021.1859476
Tafenoquine: a toxicity overview
Abstract
Introduction: A century-long history in 8-aminoquinolines, the only anti-malaria drug class preventing malaria relapse, has resulted in the approval of tafenoquine by the U.S. Food and Drug Administration (FDA) and the Australian Therapeutic Goods Administration (TGA) and to date registration in Brazil and Thailand. Tafenoquine is an alternative anti-relapse treatment for vivax malaria and malaria prophylaxis. It should not be given in pregnancy, during lactation of infants with glucose-6-phosphate dehydrogenase (G6PD) unknown or deficient status, and in those with G6PD deficiency or psychiatric illness.Areas covered: This systematic review assesses tafenoquine associated adverse events in English-language, human clinical trials. Meta-analysis of commonly reported adverse events was conducted and grouped by comparison arms.Expert opinion: Tafenoquine, either for radical cure or prophylaxis, is generally well tolerated in adults. There is no convincing evidence for neurologic, ophthalmic, and cardiac toxicities. Psychotic disorder which has been attributed to higher doses is a contraindication for the chemoprophylaxis indication and psychiatric illness is a warning for the radical cure indication. Pregnancy assessment and quantitative G6PD testing are required. The optimal radical curative regimen including the tafenoquine dose along with its safety for parts of Southeast Asia, South America, and Oceania needs further assessment.
Keywords: 8-aminoquinoline; adverse effect; causal prophylaxis; chemoprophylaxis; drug safety; meta-analysis; plasmodium vivax; radical cure; relapse prevention; tafenoquine.
Figures
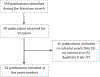



References
-
- Mühlens P Die Behandlung der natürlichen menschlichen Malaria-Infektion mit Plasmochin. Naturwissenschaften. 1926; 14:1162–1166.
-
- Zottig VE, Carr KA, Clarke JG, et al. Army antimalarial drug development: an advanced development case study for tafenoquine. Mil Med. 2020;185(Supplement_1):617–623. . - PubMed
-
-
Brueckner RP, Lasseter KC, Lin ET, et al. First-time-in-humans safety and pharmacokinetics of wr 238605, a new antimalarial. Am J Trop Med Hyg. 1998;58(5): 645–649.
• This study is the first human study characterizing tafenoquine pharmacokinetics.
-
-
- Velez ID, Tran TH, Martin A, et al. A randomized, open-label, non-comparative multicenter study to assess the pharmacokinetics, safety, and efficacy of tafenoquine in the treatment of pediatric subjects with Plasmodium vivax malaria (TEACH study). Am Soc Trop Med Hyg. 2020;427.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
