Human Lung-Resident Macrophages Colocalize with and Provide Costimulation to PD1hi Tissue-Resident Memory T Cells
- PMID: 33306940
- PMCID: PMC8456469
- DOI: 10.1164/rccm.202006-2403OC
Human Lung-Resident Macrophages Colocalize with and Provide Costimulation to PD1hi Tissue-Resident Memory T Cells
Abstract
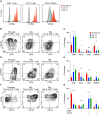
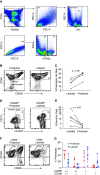
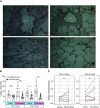
Rationale: Tissue-resident memory T cells (TRM) play a critical role in the defense against inhaled pathogens. The isolation and study of human lung tissue-resident memory T cells and lung-resident macrophages (MLR) are limited by experimental constraints. Objectives: To characterize the spatial and functional relationship between MLR and human lung tissue-resident memory T cells using ex vivo lung perfusion (EVLP). Methods: TRM and MLR were isolated using EVLP and intraperfusate-labeled CD45 antibody. Cells isolated after 6 hours of EVLP were analyzed using spectral flow cytometry. Spatial relationships between CD3+ and CD68+ cells were explored with multiplexed immunohistochemistry. Functional relationships were determined by using coculture and T-cell-receptor complex signal transduction. Measurements and Main Results: Lungs from 8 research-consenting organ donors underwent EVLP for 6 hours. We show that human lung TRM and MLR colocalize within the human lung, preferentially around the airways. Furthermore, we found that human lung CD8+ TRM are composed of two functionally distinct populations on the basis of PD1 (programed cell death receptor 1) and ZNF683 (HOBIT) protein expression. We show that MLR provide costimulatory signaling to PD1hi CD4+ and CD8+ lung TRM,, augmenting the effector cytokine production and degranulation of TRM. Conclusions: EVLP provides an innovative technique to study resident immune populations in humans. Human MLR colocalize with and provide costimulation signaling to TRM, augmenting their effector function.
Keywords: ex vivo lung perfusion; human lung immunology; innate and adaptive immune interaction; lung-resident macrophage; tissue-resident memory T cell.
Figures






Comment in
- doi: 10.1164/rccm.202012-4358ED
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

