Neutrophils Fuel Effective Immune Responses through Gluconeogenesis and Glycogenesis
- PMID: 33306983
- PMCID: PMC7863914
- DOI: 10.1016/j.cmet.2020.11.016
Neutrophils Fuel Effective Immune Responses through Gluconeogenesis and Glycogenesis
Erratum in
-
Neutrophils fuel effective immune responses through gluconeogenesis and glycogenesis.Cell Metab. 2021 May 4;33(5):1062-1064. doi: 10.1016/j.cmet.2021.03.018. Cell Metab. 2021. PMID: 33951466 Free PMC article. No abstract available.
Abstract
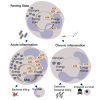
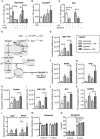
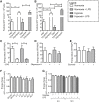
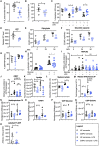
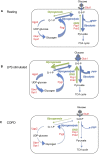
Neutrophils can function and survive in injured and infected tissues, where oxygen and metabolic substrates are limited. Using radioactive flux assays and LC-MS tracing with U-13C glucose, glutamine, and pyruvate, we observe that neutrophils require the generation of intracellular glycogen stores by gluconeogenesis and glycogenesis for effective survival and bacterial killing. These metabolic adaptations are dynamic, with net increases in glycogen stores observed following LPS challenge or altitude-induced hypoxia. Neutrophils from patients with chronic obstructive pulmonary disease have reduced glycogen cycling, resulting in impaired function. Metabolic specialization of neutrophils may therefore underpin disease pathology and allow selective therapeutic targeting.
Keywords: COPD; GYS1; gluconeogenesis; glycogen; glycogenesis; glycogenolysis; glycolysis; inflammation; neutrophil.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


References
-
- Bender J.G., Van Epps D.E. Inhibition of human neutrophil function by 6-aminonicotinamide: the role of the hexose monophosphate shunt in cell activation. Immunopharmacology. 1985;10:191–199. - PubMed
-
- Boxer L.A., Baehner R.L., Davis J. The effect of 2-deoxyglucose on guinea pig polymorphonuclear leukocyte phagocytosis. J. Cell. Physiol. 1977;91:89–102. - PubMed
-
- De Bock K., Georgiadou M., Schoors S., Kuchnio A., Wong B.W., Cantelmo A.R., Quaegebeur A., Ghesquière B., Cauwenberghs S., Eelen G. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

