The miR-199a/214 Cluster Controls Nephrogenesis and Vascularization in a Human Embryonic Stem Cell Model
- PMID: 33306987
- PMCID: PMC7897558
- DOI: 10.1016/j.stemcr.2020.11.007
The miR-199a/214 Cluster Controls Nephrogenesis and Vascularization in a Human Embryonic Stem Cell Model
Abstract
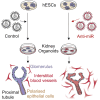
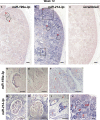
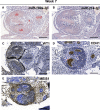
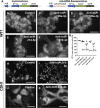
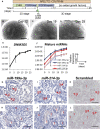
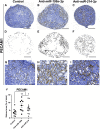
MicroRNAs (miRNAs) are gene expression regulators and they have been implicated in acquired kidney diseases and in renal development, mostly through animal studies. We hypothesized that the miR-199a/214 cluster regulates human kidney development. We detected its expression in human embryonic kidneys by in situ hybridization. To mechanistically study the cluster, we used 2D and 3D human embryonic stem cell (hESC) models of kidney development. After confirming expression in each model, we inhibited the miRNAs using lentivirally transduced miRNA sponges. This reduced the WT1+ metanephric mesenchyme domain in 2D cultures. Sponges did not prevent the formation of 3D kidney-like organoids. These organoids, however, contained dysmorphic glomeruli, downregulated WT1, aberrant proximal tubules, and increased interstitial capillaries. Thus, the miR-199a/214 cluster fine-tunes differentiation of both metanephric mesenchymal-derived nephrons and kidney endothelia. While clinical implications require further study, it is noted that patients with heterozygous deletions encompassing this miRNA locus can have malformed kidneys.
Keywords: development; human pluripotent stem cells; kidney; miR-199a; miR-214; microRNA; organoids.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Alexander M.S., Kawahara G., Motohashi N., Casar J.C., Eisenberg I., Myers J.A., Gasperini M.J., Estrella E.A., Kho A.T., Mitsuhashi S. MicroRNA-199a is induced in dystrophic muscle and affects WNT signaling, cell proliferation, and myogenic differentiation. Cell Death Differ. 2013;20:1194–1208. - PMC - PubMed
-
- Carroll T.J., Park J.S., Hayashi S., Majumdar A., McMahon A.P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell. 2005;9:283–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

