RNA therapeutics for retinal diseases
- PMID: 33307874
- PMCID: PMC8087622
- DOI: 10.1080/14712598.2021.1856365
RNA therapeutics for retinal diseases
Abstract
Introduction: In the retina, noncoding RNA (ncRNA) plays an integral role in regulating apoptosis, inflammatory responses, visual perception, and photo-transduction, with altered levels reported in diseased states.
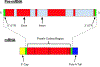
Areas covered: MicroRNA (miRNA), a class of ncRNA, regulates post-transcription gene expression through the binding of complementary sites of target messenger RNA (mRNA) with resulting translational repression. Small-interfering RNA (siRNA) is a double-stranded RNA (dsRNA) that regulates gene expression, leading to selective silencing of genes through a process called RNA interference (RNAi). Another form of RNAi involves short hairpin RNA (shRNA). In age-related macular degeneration (AMD) and diabetic retinopathy (DR), miRNA has been implicated in the regulation of angiogenesis, oxidative stress, immune response, and inflammation.
Expert opinion: Many RNA-based therapies in development are conveniently administered intravitreally, with the potential for pan-retinal effect. The majority of these RNA therapeutics are synthetic ncRNA's and hold promise for the treatment of AMD, DR, and inherited retinal diseases (IRDs). These RNA-based therapies include siRNA therapy with its high specificity, shRNA to 'knock down' autosomal dominant toxic gain of function-mutated genes, antisense oligonucleotides (ASOs), which can restore splicing defects, and translational read-through inducing drugs (TRIDs) to increase expression of full-length protein from genes with premature stop codons.
Keywords: Antisense oligonucleotides; RNA therapeutics; inherited retinal disease; microRNA; noncoding RNA; short hairpin RNA; small-interfering RNA; translational read-through inducing drugs.
Conflict of interest statement
Declaration of Interests
TA Ciulla has an employment relationship at Clearside Biomedical Inc. However, this manuscript was written during his work as a Volunteer Clinical Professor of Ophthalmology at Indiana University School of Medicine, and none of the work herein represents any official position or opinion of Clearside or its management. A Bhatwadekar reports funding support from NEI R01 EY027779. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


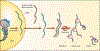
References
-
- Mercuri E, et al., Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N Engl J Med, 2018. 378(7): p. 625–635. - PubMed
-
- Heo YA, Golodirsen: First Approval. Drugs, 2020. 80(3): p. 329–333. - PubMed
-
- A randomized controlled clinical trial of intravitreous fomivirsen for treatment of newly diagnosed peripheral cytomegalovirus retinitis in patients with AIDS. Am J Ophthalmol, 2002. 133(4): p. 467–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
