Economic burden of major bleeding events in commercially insured patients with von Willebrand disease based on claims data from the United States
- PMID: 33307935
- PMCID: PMC10394209
- DOI: 10.18553/jmcp.2020.20327
Economic burden of major bleeding events in commercially insured patients with von Willebrand disease based on claims data from the United States
Abstract
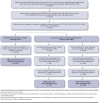
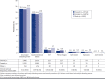
BACKGROUND: von Willebrand disease (VWD) can lead to serious, life-threatening bleeding events associated with substantial clinical and economic burden. OBJECTIVE: To estimate the prevalence, health care resource utilization (HCRU), and costs associated with major bleeding events in patients with VWD. METHODS: This was a retrospective analysis of the IBM MarketScan database (2008-2016). Selected patients had ≥ 2 VWD diagnoses, no diagnosis of acquired coagulation factor deficiency, and continuous health care plan enrollment for ≥ 12 months from eligibility start date. Prevalence was calculated as the proportion of eligible patients with ≥ 1 major bleeding event during the observation period (start to end of continuous eligibility). HCRU and costs in the 12-month continuous enrollment period following the first major bleeding event were compared with those from a comparable 12-month period for patients without major bleeding events. RESULTS: Of the 19,785 patients with VWD, 15% experienced ≥ 1 major bleeding event during a median follow-up of 4 years; 89% of these events were gastrointestinal bleeds. For the economic analysis, 773 patients with ≥ 1 major bleeding event and 4,285 patients without major bleeding events met the selection criteria. Controlling for baseline covariates, patients with major bleeding events had significantly (P < 0.0001) more inpatient admissions (incidence rate ratio [IRR] = 3.2; 95% CI = 2.78-3.77), longer inpatient stays (IRR = 3.9; 95% CI = 3.12-4.93), and more emergency department visits (IRR = 2.0; 95% CI = 1.77-2.27) and outpatient visits (IRR = 1.3; 95% CI = 1.19-1.34) than patients without major bleeding events. Annual health care costs were significantly higher (P < 0.01) for patients with major bleeding events than those without them (predicted mean cost differences: total = $20,890, pharmacy = $2,593, and medical = $18,293). CONCLUSIONS: Major bleeding events were associated with increased HCRU and costs, mostly inpatient costs. Therefore, optimizing therapy to prevent or reduce major bleeding events has the potential to reduce health care use and costs in patients with VWD. DISCLOSURES: This study was funded by Baxalta U.S. Inc., a Takeda company (Lexington, MA). The study sponsor was involved with the study design, analysis, and interpretation of data; writing of the manuscript; and the decision to publish the article. Lu, Wu, and Ewenstein are employees of Baxalta U.S. Inc., a Takeda company, and are Takeda stock owners. Farahbakhshian is an employee of Shire U.S. Inc., a Takeda company, and is a Takeda stock owner. Oladapo was an employee of Baxalta U.S. Inc., a Takeda company, at the time the analysis was completed and the manuscript developed, and is a Takeda stock owner.
Conflict of interest statement
This study was funded by Baxalta U.S. Inc., a Takeda company (Lexington, MA). The study sponsor was involved with the study design, analysis, and interpretation of data; writing of the manuscript; and the decision to publish the article. Lu, Wu, and Ewenstein are employees of Baxalta U.S. Inc., a Takeda company, and are Takeda stock owners. Farahbakhshian is an employee of Shire U.S. Inc., a Takeda company, and is a Takeda stock owner. Oladapo was an employee of Baxalta U.S. Inc., a Takeda company, at the time the analysis was completed and the manuscript developed, and is a Takeda stock owner.
Figures


References
-
- Leebeek FW, Eikenboom JC. Von Willebrand’s disease. N Engl J Med. 2016;375(21):2067-80. - PubMed
-
- Bowman M, Hopman WM, Rapson D, Lillicrap D, James P. The prevalence of symptomatic von Willebrand disease in primary care practice. J Thromb Haemost. 2010;8(1):213-16. - PubMed
-
- Nichols WL, Hultin MB, James AH, et al. Von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14(2):171-232. - PubMed
-
- Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4(10):2103-14. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

