Experience With Anti-PD-1 Antibody, Camrelizumab, Monotherapy for Biliary Tract Cancer Patients and Literature Review
- PMID: 33308041
- PMCID: PMC7739105
- DOI: 10.1177/1533033820979703
Experience With Anti-PD-1 Antibody, Camrelizumab, Monotherapy for Biliary Tract Cancer Patients and Literature Review
Abstract
Background: Novel immunotherapy is one of the options for advanced biliary tract cancer (BTC) patients who are traditionally intolerant to chemotherapy. However, clinical evidence for single immunotherapy with pembrolizumab or nivolumab is limited. The present study assessed the safety and efficiency of the anti-PD-1 antibody, camrelizumab, as monotherapy in patients with unresectable or recurrent BTC.
Methods: A retrospective evaluation was conducted among 4 patients with BTC, including 2 with intrahepatic cholangiocellular carcinoma (ICC), one with extrahepatic bile duct cancer, and one with gallbladder cancer. The patients with unresectable or recurrent BTC were refractory or intolerant to gemcitabine plus cisplatin treatment regimens and received at least one intravenous dose (3 mg/kg) of camrelizumab monotherapy every 3 weeks. Gene sequencing analysis was also performed for biomarker screening. Patient reaction was evaluated according to modified response evaluation criteria in solid tumor (RECIST) version 1.1, progression-free survival (PFS), and toxicity.
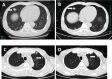
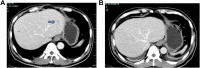
Results: In this cohort, 1 patient with recurrent ICC had a positive response to treatment, with a substantial tumor size reduction in liver and lung metastases verified using a radiological test after receiving 3 cycles of camrelizumab. The PFS was 4.9 months. The remaining 3 patients showed no response to treatment and experienced disease progression. RNA sequence analysis didn't found high expression on genes that related to PD-L1, microsatellite instability, tumor mutation burden, and DNA mismatch repair in these patients. Grade 3 treatment-related adverse event was observed in 1 patient.
Conclusions: Anti-PD-1 antibody camrelizumab had a manageable safety profile in patients with advanced BTC. This initial assessment of camrelizumab monotherapy provides effective evidence for patients with refractory BTC in biomarker-unselected patients.
Keywords: anti-PD-1; biliary tract cancer; camrelizumab; efficacy; monotherapy.
Conflict of interest statement
Figures

References
-
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

