Moving forward through the in silico modeling of tuberculosis: a further step with UISS-TB
- PMID: 33308139
- PMCID: PMC7733696
- DOI: 10.1186/s12859-020-03762-5
Moving forward through the in silico modeling of tuberculosis: a further step with UISS-TB
Abstract
Background: In 2018, about 10 million people were found infected by tuberculosis, with approximately 1.2 million deaths worldwide. Despite these numbers have been relatively stable in recent years, tuberculosis is still considered one of the top 10 deadliest diseases worldwide. Over the years, Mycobacterium tuberculosis has developed a form of resistance to first-line tuberculosis treatments, specifically to isoniazid, leading to multi-drug-resistant tuberculosis. In this context, the EU and Indian DBT funded project STriTuVaD-In Silico Trial for Tuberculosis Vaccine Development-is supporting the identification of new interventional strategies against tuberculosis thanks to the use of Universal Immune System Simulator (UISS), a computational framework capable of predicting the immunity induced by specific drugs such as therapeutic vaccines and antibiotics.
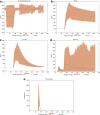
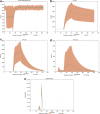
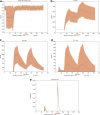
Results: Here, we present how UISS accurately simulates tuberculosis dynamics and its interaction within the immune system, and how it predicts the efficacy of the combined action of isoniazid and RUTI vaccine in a specific digital population cohort. Specifically, we simulated two groups of 100 digital patients. The first group was treated with isoniazid only, while the second one was treated with the combination of RUTI vaccine and isoniazid, according to the dosage strategy described in the clinical trial design. UISS-TB shows to be in good agreement with clinical trial results suggesting that RUTI vaccine may favor a partial recover of infected lung tissue.
Conclusions: In silico trials innovations represent a powerful pipeline for the prediction of the effects of specific therapeutic strategies and related clinical outcomes. Here, we present a further step in UISS framework implementation. Specifically, we found that the simulated mechanism of action of RUTI and INH are in good alignment with the results coming from past clinical phase IIa trials.
Keywords: Computational modeling; Immunity; In silico trials; Isoniazid; RUTI; Therapeutic strategies; Tuberculosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- WHO. Global tuberculosis report 2019. 2019.
-
- Nath H, Ryoo S. First- and second-line drugs and drug resistance. In: Tuberculosis—current issues in diagnosis and management. InTech; 2013. p. 13. 10.5772/54960.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

