PD-L1 expression in liver metastasis: its clinical significance and discordance with primary tumor in colorectal cancer
- PMID: 33308232
- PMCID: PMC7730753
- DOI: 10.1186/s12967-020-02636-x
PD-L1 expression in liver metastasis: its clinical significance and discordance with primary tumor in colorectal cancer
Abstract
Background: The outcomes of immune checkpoint inhibitors in cancer patients with liver metastases are poor, which may be related to a different tumor microenvironment in liver metastases from primary tumors. This study was aimed to analyze PD-L1 expression and the immune microenvironment status in liver metastases and compare the differences of PD-L1 expression between primary tumors and liver metastases of colorectal cancer.
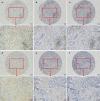
Methods: 74 cases of pathologically confirmed colorectal cancer with liver metastasis underwent resection from our hospital were included. Tissue microarrays were used for the interpretation of PD-L1 expression, cluster of differentiation 4 (CD4) and CD8 density by immunohistochemistry. We evaluated the disparity between primary tumor and liver metastasis in PD-L1 expression, CD4 and CD8 density and analyzed the factors associated with obvious PD-L1 disparity.
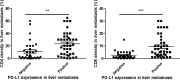
Results: The expression of PD-L1 was positively related to the density of CD4 and CD8 in liver metastases. The expression of PD-L1 in liver metastases was higher than in primary tumors in certain subgroups, including patients with concurrent liver metastases (n = 63, p = 0.05), patients receiving concurrent resection of primary and metastatic tumors (n = 56, p = 0.04). The two subgroups generally reflected those without inconsistent external influences, such as treatment and temporal factors, between primary tumors and liver metastases. In these subgroups, the intrinsic differences of microenvironment between primary tumors and liver metastases could be identified. Furthermore, tumor differentiation [moderate vs. poor: OR = 0.23, 95% CI: 0.03-0.99, p = 0.05)] were demonstrated to be associated with obvious discordance of PD-L1 expression between primary tumors and liver metastases.
Conclusions: The expression of PD-L1 in liver metastases was higher than in primary tumors in subgroups, reflecting intrinsic microenvironment differences between primary and metastatic tumors. Obvious discordance of PD-L1 expression between primary tumor and liver metastasis was significantly related to the tumor differentiation.
Keywords: Colorectal cancer; Liver metastases; PD-L1; Primary tumor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi: 10.1158/2159-8290.CD-18-0367. - DOI - PubMed
-
- Silva IPD, Lo S, Quek C, Gonzalez M, Carlino MS, Long GV, et al. Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti-PD-1 therapy. Cancer-Am Cancer Soc. 2020;126(1):86–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81930065/National Natural Science Foundation of China/International
- 2019B020227002/Science and Technology Program of Guangdong/International
- 201904020046, 201803040019, 201704020228/Science and Technology Program of Guangzhou/International
- 2019A1515110171/Guangdong Basic and Applied Basic Research Foundation/International
LinkOut - more resources
Full Text Sources
Medical
Research Materials

