Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in children and infants: two clinical trials
- PMID: 33308427
- PMCID: PMC7811205
- DOI: 10.1016/S0140-6736(20)32540-X
Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in children and infants: two clinical trials
Abstract
Background: Continued emergence and spread of circulating vaccine-derived type 2 polioviruses and vaccine-associated paralytic poliomyelitis from Sabin oral poliovirus vaccines (OPVs) has stimulated development of two novel type 2 OPV candidates (OPV2-c1 and OPV2-c2) designed to have similar immunogenicity, improved genetic stability, and less potential to reacquire neurovirulence. We aimed to assess safety and immunogenicity of the two novel OPV candidates compared with a monovalent Sabin OPV in children and infants.
Methods: We did two single-centre, multi-site, partly-masked, randomised trials in healthy cohorts of children (aged 1-4 years) and infants (aged 18-22 weeks) in Panama: a control phase 4 study with monovalent Sabin OPV2 before global cessation of monovalent OPV2 use, and a phase 2 study with low and high doses of two novel OPV2 candidates. All participants received one OPV2 vaccination and subsets received two doses 28 days apart. Parents reported solicited and unsolicited adverse events. Type 2 poliovirus neutralising antibodies were measured at days 0, 7, 28, and 56, and stool viral shedding was assessed up to 28 days post-vaccination. Primary objectives were to assess safety in all participants and non-inferiority of novel OPV2 day 28 seroprotection versus monovalent OPV2 in infants (non-inferiority margin 10%). These studies were registered with ClinicalTrials.gov, NCT02521974 and NCT03554798.
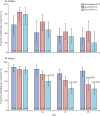
Findings: The control study took place between Oct 23, 2015, and April 29, 2016, and the subsequent phase 2 study between Sept 19, 2018, and Sept 30, 2019. 150 children (50 in the control study and 100 of 129 assessed for eligibility in the novel OPV2 study) and 684 infants (110 of 114 assessed for eligibility in the control study and 574 of 684 assessed for eligibility in the novel OPV2 study) were enrolled and received at least one study vaccination. Vaccinations were safe and well tolerated with no causally associated serious adverse events or important medical events in any group. Solicited and unsolicited adverse events were overwhelmingly mild or moderate irrespective of vaccine or dose. Nearly all children were seroprotected at baseline, indicating high baseline immunity. In children, the seroprotection rate 28 days after one dose was 100% for monovalent OPV2 and both novel OPV2 candidates. In infants at day 28, 91 (94% [95% CI 87-98]) of 97 were seroprotected after receiving monovalent OPV2, 134 (94% [88-97]) of 143 after high-dose novel OPV2-c1, 122 (93% [87-97]) of 131 after low-dose novel OPV2-c1, 138 (95% [90-98]) of 146 after high-dose novel OPV2-c2, and 115 (91% [84-95]) of 127 after low-dose novel OPV2-c2. Non-inferiority was shown for low-dose and high-dose novel OPV2-c1 and high-dose novel OPV2-c2 despite monovalent OPV2 recipients having higher baseline immunity.
Interpretation: Both novel OPV2 candidates were safe, well tolerated, and immunogenic in children and infants. Novel OPV2 could be an important addition to our resources against poliovirus given the current epidemiological situation.
Funding: Fighting Infectious Diseases in Emerging Countries and Bill & Melinda Gates Foundation.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Poliovirus vaccine options: another step forward.Lancet. 2021 Jan 2;397(10268):2-3. doi: 10.1016/S0140-6736(20)32629-5. Epub 2020 Dec 9. Lancet. 2021. PMID: 33308426 No abstract available.
References
-
- WHO Two out of three wild poliovirus strains eradicated. 2019. https://www.who.int/news-room/feature-stories/detail/two-out-of-three-wi...
-
- Global Polio Eradication Initiative Polio this week as of 03 December 2020. 2020. http://polioeradication.org/wp-content/uploads/2020/12/weekly-polio-anal...
-
- WHO Meeting of the strategic advisory group of experts on immunization, 31 March–1 April 2020: conclusions and recommendations: polio. Wkly Epidemiol Rec. 2020;22:249–252.
-
- Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-derived polioviruses. J Infect Dis. 2014;210(suppl 1):S283–S293. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

