Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in healthy adults: two clinical trials
- PMID: 33308429
- PMCID: PMC7811203
- DOI: 10.1016/S0140-6736(20)32541-1
Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in healthy adults: two clinical trials
Abstract
Background: Two novel type 2 oral poliovirus vaccine (OPV2) candidates, novel OPV2-c1 and novel OPV2-c2, designed to be more genetically stable than the licensed Sabin monovalent OPV2, have been developed to respond to ongoing polio outbreaks due to circulating vaccine-derived type 2 polioviruses.
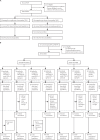
Methods: We did two randomised studies at two centres in Belgium. The first was a phase 4 historical control study of monovalent OPV2 in Antwerp, done before global withdrawal of OPV2, and the second was a phase 2 study in Antwerp and Ghent with novel OPV2-c1 and novel OPV2-c2. Eligible participants were healthy adults aged 18-50 years with documented history of at least three polio vaccinations, including OPV in the phase 4 study and either OPV or inactivated poliovirus vaccine (IPV) in the novel OPV2 phase 2 study, with no dose within 12 months of study start. In the historical control trial, participants were randomly assigned to either one dose or two doses of monovalent OPV2. In the novel OPV2 trial, participants with previous OPV vaccinations were randomly assigned to either one or two doses of novel OPV2-c1 or to one or two doses of novel OPV2-c2. IPV-vaccinated participants were randomly assigned to receive two doses of either novel OPV2-c1, novel OPV2-c2, or placebo. Vaccine administrators were unmasked to treatment; medical staff performing safety and reactogenicity assessments or blood draws for immunogenicity assessments were masked. Participants received the first vaccine dose on day 0, and a second dose on day 28 if assigned to receive a second dose. Primary objectives were assessments and comparisons of safety up to 28 days after each dose, including solicited adverse events and serious adverse events, and immunogenicity (seroprotection rates on day 28 after the first vaccine dose) between monovalent OPV2 and the two novel OPV2 candidates. Primary immunogenicity analyses were done in the per-protocol population. Safety was assessed in the total vaccinated population-ie, all participants who received at least one dose of their assigned vaccine. The phase 4 control study is registered with EudraCT (2015-003325-33) and the phase 2 novel OPV2 study is registered with EudraCT (2018-001684-22) and ClinicalTrials.gov (NCT04544787).
Findings: In the historical control study, between Jan 25 and March 18, 2016, 100 volunteers were enrolled and randomly assigned to receive one or two doses of monovalent OPV2 (n=50 in each group). In the novel OPV2 study, between Oct 15, 2018, and Feb 27, 2019, 200 previously OPV-vaccinated volunteers were assigned to the four groups to receive one or two doses of novel OPV2-c1 or novel OPV2-c2 (n=50 per group); a further 50 participants, previously vaccinated with IPV, were assigned to novel OPV2-c1 (n=17), novel OPV2-c2 (n=16), or placebo (n=17). All participants received the first dose of assigned vaccine or placebo and were included in the total vaccinated population. All vaccines appeared safe; no definitely vaccine-related withdrawals or serious adverse events were reported. After first doses in previously OPV-vaccinated participants, 62 (62%) of 100 monovalent OPV2 recipients, 71 (71%) of 100 recipients of novel OPV2-c1, and 74 (74%) of 100 recipients of novel OPV2-c2 reported solicited systemic adverse events, four (monovalent OPV2), three (novel OPV2-c1), and two (novel OPV2-c2) of which were considered severe. In IPV-vaccinated participants, solicited adverse events occurred in 16 (94%) of 17 who received novel OPV2-c1 (including one severe) and 13 (81%) of 16 who received novel OPV2-c2 (including one severe), compared with 15 (88%) of 17 placebo recipients (including two severe). In previously OPV-vaccinated participants, 286 (97%) of 296 were seropositive at baseline; after one dose, 100% of novel OPV2 vaccinees and 97 (97%) of monovalent OPV2 vaccinees were seropositive.
Interpretation: Novel OPV2 candidates were as safe, well tolerated, and immunogenic as monovalent OPV2 in previously OPV-vaccinated and IPV-vaccinated adults. These data supported the further assessment of the vaccine candidates in children and infants.
Funding: University of Antwerp and Bill & Melinda Gates Foundation.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Poliovirus vaccine options: another step forward.Lancet. 2021 Jan 2;397(10268):2-3. doi: 10.1016/S0140-6736(20)32629-5. Epub 2020 Dec 9. Lancet. 2021. PMID: 33308426 No abstract available.
References
-
- WHO Two out of three wild poliovirus strains eradicated. Oct 24, 2019. https://www.who.int/news-room/feature-stories/detail/two-out-of-three-wi...
-
- Global Polio Eradication Initiative Polio this week as of 25 November 2020. Nov 25, 2020. http://polioeradication.org/polio-today/polio-now/this-week/
-
- WHO Polio. Wkly Epidemiol Rec. 2019;95:249–252.
-
- Global Polio Eradication Initiative . World Health Organization; Geneva: 2020. Strategy for the response to type 2 circulating vaccine-derived poliovirus 2020–2021: an addendum to the Polio Endgame Strategy 2019–2023.https://polioeradication.org/wp-content/uploads/2020/04/Strategy-for-the...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous