SCUBE3 loss-of-function causes a recognizable recessive developmental disorder due to defective bone morphogenetic protein signaling
- PMID: 33308444
- PMCID: PMC7820739
- DOI: 10.1016/j.ajhg.2020.11.015
SCUBE3 loss-of-function causes a recognizable recessive developmental disorder due to defective bone morphogenetic protein signaling
Abstract
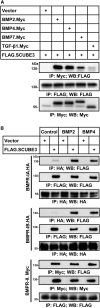
Signal peptide-CUB-EGF domain-containing protein 3 (SCUBE3) is a member of a small family of multifunctional cell surface-anchored glycoproteins functioning as co-receptors for a variety of growth factors. Here we report that bi-allelic inactivating variants in SCUBE3 have pleiotropic consequences on development and cause a previously unrecognized syndromic disorder. Eighteen affected individuals from nine unrelated families showed a consistent phenotype characterized by reduced growth, skeletal features, distinctive craniofacial appearance, and dental anomalies. In vitro functional validation studies demonstrated a variable impact of disease-causing variants on transcript processing, protein secretion and function, and their dysregulating effect on bone morphogenetic protein (BMP) signaling. We show that SCUBE3 acts as a BMP2/BMP4 co-receptor, recruits the BMP receptor complexes into raft microdomains, and positively modulates signaling possibly by augmenting the specific interactions between BMPs and BMP type I receptors. Scube3-/- mice showed craniofacial and dental defects, reduced body size, and defective endochondral bone growth due to impaired BMP-mediated chondrogenesis and osteogenesis, recapitulating the human disorder. Our findings identify a human disease caused by defective function of a member of the SCUBE family, and link SCUBE3 to processes controlling growth, morphogenesis, and bone and teeth development through modulation of BMP signaling.
Keywords: BMP; BMP receptors; SCUBE; bone morphogenetic protein; genomic sequencing; intracellular signaling; mechanism of disease; morphogenesis; skeletal development.
Copyright © 2020 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
C.B., P.B., and N.-M.G. declare no additional conflicts of interest beyond their employment affiliation. All the other authors declare no competing interests.
Figures






References
-
- Salazar V.S., Gamer L.W., Rosen V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016;12:203–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

