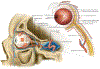
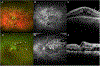
Retinal vascular occlusions
- PMID: 33308475
- PMCID: PMC9546635
- DOI: 10.1016/S0140-6736(20)31559-2
Retinal vascular occlusions
Abstract
Acute retinal vascular occlusions are common causes of visual impairment. Although both retinal artery occlusions and retinal vein occlusions are associated with increased age and cardiovascular risk factors, their pathophysiology, systemic implications, and management differ substantially. Acute management of retinal artery occlusions involves a multidisciplinary approach including neurologists with stroke expertise, whereas treatment of retinal vein occlusions is provided by ophthalmologists. Optimisation of systemic risk factors by patients' primary care providers is an important component of the management of these two disorders.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures


References
-
- Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol 1997; 115: 486–91. - PubMed
-
- Ponto KA, Scharrer I, Binder H, et al. Hypertension and multiple cardiovascular risk factors increase the risk for retinal vein occlusions: results from the Gutenberg Retina lVein Occlusion Study J Hypertension 2019; 37: 1372–83. - PubMed
-
- Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia. Follow the guidelines! Ophthalmology 2018; 125: 1597–607. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

