Sex Differences in Nociceptor Translatomes Contribute to Divergent Prostaglandin Signaling in Male and Female Mice
- PMID: 33309016
- PMCID: PMC8019688
- DOI: 10.1016/j.biopsych.2020.09.022
Sex Differences in Nociceptor Translatomes Contribute to Divergent Prostaglandin Signaling in Male and Female Mice
Abstract
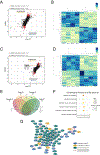
Background: There are clinically relevant sex differences in acute and chronic pain mechanisms, but we are only beginning to understand their mechanistic basis. Transcriptome analyses of rodent whole dorsal root ganglion (DRG) have revealed sex differences, mostly in immune cells. We examined the transcriptome and translatome of the mouse DRG with the goal of identifying sex differences.
Methods: We used translating ribosome affinity purification sequencing and behavioral pharmacology to test the hypothesis that in Nav1.8-positive neurons, most of which are nociceptors, translatomes would differ by sex.
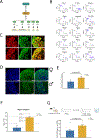
Results: We found 80 genes with sex differential expression in the whole DRG transcriptome and 66 genes whose messenger RNAs were sex differentially actively translated (translatome). We also identified different motifs in the 3' untranslated region of messenger RNAs that were sex differentially translated. In further validation studies, we focused on Ptgds, which was increased in the translatome of female mice. The messenger RNA encodes the prostaglandin PGD2 synthesizing enzyme. We observed increased PTGDS protein and PGD2 in female mouse DRG. The PTGDS inhibitor AT-56 caused intense pain behaviors in male mice but was only effective at high doses in female mice. Conversely, female mice responded more robustly to another major prostaglandin, PGE2, than did male mice. PTGDS protein expression was also higher in female cortical neurons, suggesting that DRG findings may be generalizable to other nervous system structures.
Conclusions: Our results demonstrate sex differences in nociceptor-enriched translatomes and reveal unexpected sex differences in one of the oldest known nociceptive signaling molecule families, the prostaglandins.
Keywords: Nociceptor; PGD2; PGE2; PTGDS; Pain; Prostaglandins; Sex differences; Translating ribosome affinity purification.
Copyright © 2020 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Mendrek A, Mancini-Marïe A (2016): Sex/gender differences in the brain and cognition in schizophrenia. Neurosci Biobehav Rev. 67:57–78. - PubMed
-
- Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, et al. (2000): Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology. 55:1358–1363. - PubMed
-
- Georgiev D, Hamberg K, Hariz M, Forsgren L, Hariz GM (2017): Gender differences in Parkinson’s disease: A clinical perspective. Acta Neurol Scand. 136:570–584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

