Nucleic Acid-Based Technologies Targeting Coronaviruses
- PMID: 33309323
- PMCID: PMC7691141
- DOI: 10.1016/j.tibs.2020.11.010
Nucleic Acid-Based Technologies Targeting Coronaviruses
Abstract
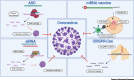
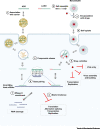
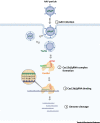
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is currently creating a global health emergency. This crisis is driving a worldwide effort to develop effective vaccines, prophylactics, and therapeutics. Nucleic acid (NA)-based treatments hold great potential to combat outbreaks of coronaviruses (CoVs) due to their rapid development, high target specificity, and the capacity to increase druggability. Here, we review key anti-CoV NA-based technologies, including antisense oligonucleotides (ASOs), siRNAs, RNA-targeting clustered regularly interspaced short palindromic repeats-CRISPR-associated protein (CRISPR-Cas), and mRNA vaccines, and discuss improved delivery methods and combination therapies with other antiviral drugs.
Keywords: RNA-targeting CRISPR-Cas; antisense oligonucleotide (ASO); coronavirus (CoV); lipid-ASO nanomicelle; mRNA vaccines; siRNA.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

