Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies
- PMID: 33309601
- PMCID: PMC8005435
- DOI: 10.1016/j.molmed.2020.11.006
Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies
Abstract
Leakage from blood vessels into tissues is governed by mechanisms that control endothelial barrier function to maintain homeostasis. Dysregulated endothelial permeability contributes to many conditions and can influence disease morbidity and treatment. Diverse approaches used to study endothelial permeability have yielded a wealth of valuable insights. Yet, ongoing questions, technical challenges, and unresolved controversies relating to the mechanisms and relative contributions of barrier regulation, transendothelial sieving, and transport of fluid, solutes, and particulates complicate interpretations in the context of vascular physiology and pathophysiology. Here, we describe recent in vivo findings and other advances in understanding endothelial barrier function with the goal of identifying and reconciling controversies over cellular and molecular processes that regulate the vascular barrier in health and disease.
Keywords: G protein-coupled receptors; Rho GTPases; VEGF receptors; endothelial barrier function; endothelial cell junctions; gap formation; postcapillary venules.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Figures


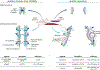
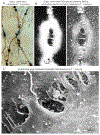

References
-
- Stan RV (2006) Channels across endothelial cells. In Cell-Cell Channels (Baluska F. et al. eds), pp. 271–278, Landes Bioscience, Georgetown, TX, USA.
-
- Michel CC (1997) Starling: the formulation of his hypothesis of microvascular fluid exchange and its significance after 100 years. Exp Physiol 82 (1), 1–30. - PubMed
-
- Palade GE et al. (1979) Structural aspects of the permeability of the microvascular endothelium. Acta Physiol Scand Suppl 463, 11–32. - PubMed
-
- Simionescu M. and Simionescu N. (1984) Ultrastructure of the microvascular wall: functional correlations. Chapter 3. In Handbook of Physiology (Renkin EM and Michel CC eds), pp. 41–101, American Physiological Society.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

