KCNV2-Associated Retinopathy: Genetics, Electrophysiology, and Clinical Course-KCNV2 Study Group Report 1
- PMID: 33309813
- PMCID: PMC8186730
- DOI: 10.1016/j.ajo.2020.11.022
KCNV2-Associated Retinopathy: Genetics, Electrophysiology, and Clinical Course-KCNV2 Study Group Report 1
Abstract
Purpose: To investigate genetics, electrophysiology, and clinical course of KCNV2-associated retinopathy in a cohort of children and adults.
Study design: This was a multicenter international clinical cohort study.
Methods: Review of clinical notes and molecular genetic testing. Full-field electroretinography (ERG) recordings, incorporating the international standards, were reviewed and quantified and compared with age and recordings from control subjects.
Results: In total, 230 disease-associated alleles were identified from 117 patients, corresponding to 75 different KCNV2 variants, with 28 being novel. The mean age of onset was 3.9 years old. All patients were symptomatic before 12 years of age (range, 0-11 years). Decreased visual acuity was present in all patients, and 4 other symptoms were common: reduced color vision (78.6%), photophobia (53.5%), nyctalopia (43.6%), and nystagmus (38.6%). After a mean follow-up of 8.4 years, the mean best-corrected visual acuity (BCVA ± SD) decreased from 0.81 ± 0.27 to 0.90 ± 0.31 logarithm of minimal angle of resolution. Full-field ERGs showed pathognomonic waveform features. Quantitative assessment revealed a wide range of ERG amplitudes and peak times, with a mean rate of age-associated reduction indistinguishable from the control group. Mean amplitude reductions for the dark-adapted 0.01 ERG, dark-adapted 10 ERG a-wave, and LA 3.0 30 Hz and LA3 ERG b-waves were 55%, 21%, 48%, and 74%, respectively compared with control values. Peak times showed stability across 6 decades.
Conclusion: In KCNV2-associated retinopathy, full-field ERGs are diagnostic and consistent with largely stable peripheral retinal dysfunction. Report 1 highlights the severity of the clinical phenotype and established a large cohort of patients, emphasizing the unmet need for trials of novel therapeutics.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
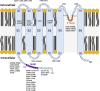
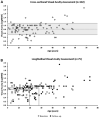


References
-
- Gouras P., Eggers H.M., MacKay C.J. Cone dystrophy, nyctalopia, and supernormal rod responses. A new retinal degeneration. Arch Ophthalmol. 1983;101(5):718–724. - PubMed
-
- Czirjak G., Toth Z.E., Enyedi P. Characterization of the heteromeric potassium channel formed by kv2.1 and the retinal subunit kv8.2 in Xenopus oocytes. J Neurophysiol. 2007;98(3):1213–1222. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

