Microglial vesicles improve post-stroke recovery by preventing immune cell senescence and favoring oligodendrogenesis
- PMID: 33309882
- PMCID: PMC8058432
- DOI: 10.1016/j.ymthe.2020.12.009
Microglial vesicles improve post-stroke recovery by preventing immune cell senescence and favoring oligodendrogenesis
Abstract
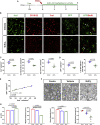
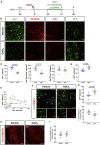
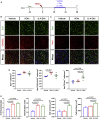
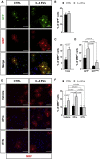
Contrasting myelin damage through the generation of new myelinating oligodendrocytes represents a promising approach to promote functional recovery after stroke. Here, we asked whether activation of microglia and monocyte-derived macrophages affects the regenerative process sustained by G protein-coupled receptor 17 (GPR17)-expressing oligodendrocyte precursor cells (OPCs), a subpopulation of OPCs specifically reacting to ischemic injury. GPR17-iCreERT2:CAG-eGFP reporter mice were employed to trace the fate of GPR17-expressing OPCs, labeled by the green fluorescent protein (GFP), after permanent middle cerebral artery occlusion. By microglia/macrophages pharmacological depletion studies, we show that innate immune cells favor GFP+ OPC reaction and limit myelin damage early after injury, whereas they lose their pro-resolving capacity and acquire a dystrophic "senescent-like" phenotype at later stages. Intracerebral infusion of regenerative microglia-derived extracellular vesicles (EVs) restores protective microglia/macrophages functions, limiting their senescence during the post-stroke phase, and enhances the maturation of GFP+ OPCs at lesion borders, resulting in ameliorated neurological functionality. In vitro experiments show that EV-carried transmembrane tumor necrosis factor (tmTNF) mediates the pro-differentiating effects on OPCs, with future implications for regenerative therapies.
Keywords: GPR17 receptor; TNF; cerebral ischemia; extracellular vesicles; functional recovery; microglia; neuroinflammation; oligodendrocyte precursor cells; remyelination; tumor necrosis factor.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R. Heart Disease and Stroke Statistics−2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. - PubMed
-
- Rost N.S., Cougo P., Lorenzano S., Li H., Cloonan L., Bouts M.J.R.J., Lauer A., Etherton M.R., Karadeli H.H., Musolino P.L. Diffuse microvascular dysfunction and loss of white matter integrity predict poor outcomes in patients with acute ischemic stroke. J. Cereb. Blood Flow Metab. 2018;38:75–86. - PMC - PubMed
-
- Marshall R.S. Progress in intravenous thrombolytic therapy for acute stroke. JAMA Neurol. 2015;72:928–934. - PubMed

