Conditional promoters to investigate gene function during wheat infection by Zymoseptoria tritici
- PMID: 33309991
- PMCID: PMC7812376
- DOI: 10.1016/j.fgb.2020.103487
Conditional promoters to investigate gene function during wheat infection by Zymoseptoria tritici
Abstract
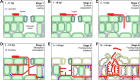
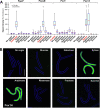
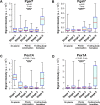
The fungus Zymoseptoria tritici causes Septoria tritici leaf blotch, which poses a serious threat to temperate-grown wheat. Recently, we described a raft of molecular tools to study the biology of this fungus in vitro. Amongst these are 5 conditional promoters (Pnar1, Pex1A, Picl1, Pgal7, PlaraB), which allow controlled over-expression or repression of target genes in cells grown in liquid culture. However, their use in the host-pathogen interaction in planta was not tested. Here, we investigate the behaviour of these promoters by quantitative live cell imaging of green-fluorescent protein-expressing cells during 6 stages of the plant infection process. We show that Pnar1 and Picl1 are repressed in planta and demonstrate their suitability for studying essential gene expression and function in plant colonisation. The promoters Pgal7 and Pex1A are not fully-repressed in planta, but are induced during pycnidiation. This indicates the presence of inducing galactose or xylose and/or arabinose, released from the plant cell wall by the activity of fungal hydrolases. In contrast, the PlaraB promoter, which normally controls expression of an α-l-arabinofuranosidase B, is strongly induced inside the leaf. This suggests that the fungus is exposed to L-arabinose in the mesophyll apoplast. Taken together, this study establishes 2 repressible promoters (Pnar1 and Picl1) and three inducible promoters (Pgal7, Pex1A, PlaraB) for molecular studies in planta. Moreover, we provide circumstantial evidence for plant cell wall degradation during the biotrophic phase of Z. tritici infection.
Keywords: Conditional promoters; Plant cell wall-degrading enzymes; Plant-pathogen interaction; Septoria tritici leaf blotch.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Bottin A., Kämper J., Kahmann R. Isolation of a carbon source-regulated gene from Ustilago maydis. Mol. Gen. Genet. 1996;253:342–352. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

