Clinical evaluation of commercial automated SARS-CoV-2 immunoassays
- PMID: 33310108
- PMCID: PMC7725057
- DOI: 10.1016/j.ijid.2020.12.003
Clinical evaluation of commercial automated SARS-CoV-2 immunoassays
Abstract
Objective: Numerous immunoassays for detecting antibodies directed against SARS-CoV-2 have been rapidly developed and released. Validations of these have been performed with a limited number of samples. The lack of standardisation might lead to significantly different results. This study compared ten automated assays from six vendors in terms of sensitivity, specificity and reproducibility.
Methods: This study compared ten fully automated immunoassays from the following vendors: Diasorin, Epitope Diagnostics, Euroimmun, Roche, YHLO, and Snibe. The retrospective part of the study included patients with a laboratory-confirmed COVID-19 infection, and controls comprised patients with a suspected infection, in whom the disease was excluded. Furthermore, biobanked sera were taken as negative controls (n = 97). The retrospective part involved four groups: (1) laboratory-confirmed COVID-19 infection (n = 183); (1B) suspected COVID-19 infection (n = 167) without a qRT-PCR result but positive serological results from at least two different assays, and suspected COVID-19 infection due to a positive serological result from the Roche assay (n = 295); (2) biobanked sera obtained from patients before the emergence of SARS-CoV-2 (n = 97) as negative controls; and (2A) probably COVID-19-negative sera with negative serological results from at least two different assays (n = 152).
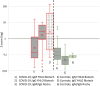
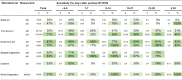
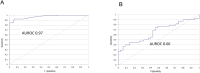
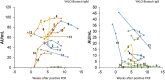
Results: Overall diagnostic sensitivities were: Euroimmun (IgA) 87%; Epitope Diagnostics (IgG) 83%; YHLO (IgG) 77%; Roche (IgM/IgG) 77%; Euroimmun (IgG) 75%; Diasorin (IgG) 53%; Epitope Diagnostics (IgM) 52%; Snibe (IgG) 47%; YHLO (IgM) 35%; and Snibe (IgM) 26%. Diagnostic specificities were: YHLO (IgG) 100%; Roche, 100%; Snibe (IgM/IgG) 100%; Diasorin (IgG) 97%; Euroimmun (IgG) 94%; YHLO (IgM) 94%; Euroimmun (IgA) 83%.
Conclusion: Assays from different vendors substantially varied in terms of their performance. These findings might facilitate selection of appropriate serological assays.
Keywords: Automated SARS-CoV-2 antibody detection; High throughpu testing; Longitudional monitoring of antibody development; Pandemic control; Seroconversion; Serpprevalence.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Bryant J.E., Azman A.S., Ferrari M.J., Arnold B.F., Boni M.F., Boum Y., et al. Serology for SARS-CoV-2: apprehensions, opportunities, and the path forward. Sci Immunol. 2020;5(47) - PubMed
-
- Bundesärztekammer Z.E.bd. Die (Weiter-)Verwendung von menschlichen Körpermaterialien für Zwecke medizinischer Forschung. Dtsch Arztebl. 2003;100(23):A1632.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

