Ozone therapy for patients with COVID-19 pneumonia: Preliminary report of a prospective case-control study
- PMID: 33310665
- PMCID: PMC7833586
- DOI: 10.1016/j.intimp.2020.107261
Ozone therapy for patients with COVID-19 pneumonia: Preliminary report of a prospective case-control study
Abstract
Background: There is still no specific treatment strategies for COVID-19 other than supportive management.
Design: A prospective case-control study determined by admittance to the hospital based on bed availability.
Participants: Eighteen patients with COVID-19 infection (laboratory confirmed) severe pneumonia admitted to hospital between 20th March and 19th April 2020. Patients admitted to the hospital during the study period were assigned to different beds based on bed availability. Depending on the bed the patient was admitted, the treatment was ozone autohemotherapy or standard treatment. Patients in the case group received ozonated blood twice daily starting on the day of admission for a median of four days. Each treatment involved administration of 200 mL autologous whole blood enriched with 200 mL of oxygen-ozone mixture with a 40 μg/mL ozone concentration.
Main outcomes: The primary outcome was time from hospital admission to clinical improvement.
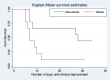
Results: Nine patients (50%) received ozonated autohemotherapy beginning on the day of admission. Ozonated autohemotherapy was associated with shorter time to clinical improvement (median [IQR]), 7 days [6-10] vs 28 days [8-31], p = 0.04) and better outcomes at 14-days (88.8% vs 33.3%, p = 0.01). In risk-adjusted analyses, ozonated autohemotherapy was associated with a shorter mean time to clinical improvement (-11.3 days, p = 0.04, 95% CI -22.25 to -0.42).
Conclusion: Ozonated autohemotherapy was associated with a significantly shorter time to clinical improvement in this prospective case-control study. Given the small sample size and study design, these results require evaluation in larger randomized controlled trials.
Clinical trial registration number: NCT04444531.
Keywords: COVID-19; Oxygen-ozone therapy; Ozonated blood; Pneumonia; Time to clinical improvement.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
- Johns Hopkins University and Medicine; COVID-19 map Johns Hopkins Coronavirus Resource Centre, https://coronavirus.jhu.edu/map.html.
-
- Giunta R., Coppola A., Luongo C., et al. Ozonized autohemotransfusion improves hemorheological parameters and oxygen delivery to tissues in patients with peripheral occlusive arterial disease. Ann. Hematol. 2001;80(12):745–748. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous