Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2
- PMID: 33310900
- PMCID: PMC7817128
- DOI: 10.1073/pnas.2021450118
Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2
Abstract
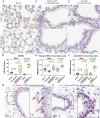
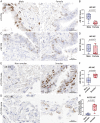
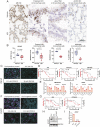
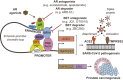
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19, employs two key host proteins to gain entry and replicate within cells, angiotensin-converting enzyme 2 (ACE2) and the cell surface transmembrane protease serine 2 (TMPRSS2). TMPRSS2 was first characterized as an androgen-regulated gene in the prostate. Supporting a role for sex hormones, males relative to females are disproportionately affected by COVID-19 in terms of mortality and morbidity. Several studies, including one employing a large epidemiological cohort, suggested that blocking androgen signaling is protective against COVID-19. Here, we demonstrate that androgens regulate the expression of ACE2, TMPRSS2, and androgen receptor (AR) in subsets of lung epithelial cells. AR levels are markedly elevated in males relative to females greater than 70 y of age. In males greater than 70 y old, smoking was associated with elevated levels of AR and ACE2 in lung epithelial cells. Transcriptional repression of the AR enhanceosome with AR or bromodomain and extraterminal domain (BET) antagonists inhibited SARS-CoV-2 infection in vitro. Taken together, these studies support further investigation of transcriptional inhibition of critical host factors in the treatment or prevention of COVID-19.
Keywords: ACE2; BET inhibitors; SARS-CoV-2; TMPRSS2; androgen receptor.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Wiersinga W. J., Rhodes A., Cheng A. C., Peacock S. J., Prescott H. C., Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 324, 782–793 (2020). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

