The neuroanatomical-functional paradox in spinal cord injury
- PMID: 33311711
- PMCID: PMC9012488
- DOI: 10.1038/s41582-020-00436-x
The neuroanatomical-functional paradox in spinal cord injury
Erratum in
-
Publisher Correction: The neuroanatomical-functional paradox in spinal cord injury.Nat Rev Neurol. 2023 Oct;19(10):635. doi: 10.1038/s41582-023-00865-4. Nat Rev Neurol. 2023. PMID: 37553394 No abstract available.
Abstract
Although lesion size is widely considered to be the most reliable predictor of outcome after CNS injury, lesions of comparable size can produce vastly different magnitudes of functional impairment and subsequent recovery. This neuroanatomical-functional paradox is likely to contribute to the many failed attempts to independently replicate findings from animal models of neurotrauma. In humans, the analogous clinical-radiological paradox could explain why individuals with similar injuries can respond differently to rehabilitation. We describe the neuroanatomical-functional paradox in the context of traumatic spinal cord injury (SCI) and discuss the underlying mechanisms of the paradox, including the concepts of lesion-affected and recovery-related networks. We also consider the various secondary complications that further limit the accuracy of outcome prediction in SCI and provide suggestions for how to increase the predictive, translational value of preclinical SCI models.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

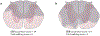


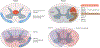

References
-
- Marino RJ, Ditunno JF Jr., Donovan WH & Maynard F Jr. Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch. Phys. Med. Rehabil 80, 1391–1396 (1999). - PubMed
-
- Fawcett JW et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45, 190–205 (2007). - PubMed
-
- Schucht P, Raineteau O, Schwab ME & Fouad K Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol 176, 143–153 (2002). - PubMed
-
- Hurd C, Weishaupt N & Fouad K Anatomical correlates of recovery in single pellet reaching in spinal cord injured rats. Exp. Neurol 247, 605–614 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

