Prognostic value of changes in serum carcinoembryonic antigen levels for preoperative chemoradiotherapy response in locally advanced rectal cancer
- PMID: 33311947
- PMCID: PMC7701949
- DOI: 10.3748/wjg.v26.i44.7022
Prognostic value of changes in serum carcinoembryonic antigen levels for preoperative chemoradiotherapy response in locally advanced rectal cancer
Abstract
Background: Preoperative chemoradiotherapy (CRT) is a standard treatment modality for locally advanced rectal cancer. However, CRT alone cannot improve overall survival. Approximately 20% of patients with CRT-resistant tumors show disease progression. Therefore, predictive factors for treatment response are needed to identify patients who will benefit from CRT. We theorized that the prognosis may vary if patients are classified according to pre- to post-CRT changes in carcinoembryonic antigen (CEA) levels.
Aim: To identify patients with locally advanced rectal cancer for preoperative chemoradiotherapy based on carcinoembryonic antigen levels.
Methods: We retrospectively included locally advanced rectal cancer patients who underwent preoperative CRT and curative resection between 2011 and 2017. Patients were assigned to groups A, B, and C based on pre- and post-CRT serum CEA levels: Both > 5; pre > 5 and post ≤ 5; and both ≤ 5 ng/mL, respectively. We compared the response to CRT based on changes in serum CEA levels. Receiver operating characteristic curve analysis was performed to determine optimal cutoff for neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Multivariate logistic regression analysis was used to evaluate the prognostic factors for pathologic complete response (pCR)/good response.
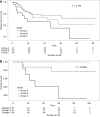
Results: The cohort comprised 145 patients; of them, 27, 43, and 65 belonged to groups A, B, and C, respectively, according to changes in serum CEA levels before and after CRT. Pre- (P < 0.001) and post-CRT (P < 0.001) CEA levels and the ratio of down-staging (P = 0.013) were higher in Groups B and C than in Group A. The ratio of pathologic tumor regression grade 0/1 significantly differed among the groups (P = 0.003). Group C had the highest number of patients showing pCR (P < 0.001). Most patients with pCR showed pre- and post-CRT CEA levels < 5 ng/mL (P < 0.001, P = 0.008). Pre- and post-CRT CEA levels were important risk factors for pCR (OR = 18.71; 95%CI: 4.62-129.51, P < 0.001) and good response (OR = 5.07; 95%CI: 1.92-14.83, P = 0.002), respectively. Pre-CRT neutrophil-lymphocyte ratio and post-CRT T ≥ 3 stage were also prognostic factors for pCR or good response.
Conclusion: Pre- and post-CRT CEA levels, as well as change in CEA levels, were prognostic markers for treatment response to CRT and may facilitate treatment individualization for rectal cancer.
Keywords: Carcinoembryonic antigen levels; Change in serum carcinoembryonic antigen; Neoadjuvant chemoradiation therapy; Prognostic factor; Rectal cancer; Response of chemoradiotherapy.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: There are no conflicts of interest to declare.
Figures
References
-
- Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R German Rectal Cancer Study Group. Preoperative vs postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. - PubMed
-
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. - PubMed
-
- Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, Seitz JF, Buecher B, Mackiewicz R, Ducreux M, Bedenne L. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. - PubMed
-
- Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, Radosevic-Jelic L, Piérart M, Calais G European Organisation for Research and Treatment of Cancer Radiation Oncology Group. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? J Clin Oncol. 2007;25:4379–4386. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

