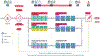I-SPY 2: a Neoadjuvant Adaptive Clinical Trial Designed to Improve Outcomes in High-Risk Breast Cancer
- PMID: 33312344
- PMCID: PMC7731787
- DOI: 10.1007/s12609-019-00334-2
I-SPY 2: a Neoadjuvant Adaptive Clinical Trial Designed to Improve Outcomes in High-Risk Breast Cancer
Abstract
Purpose of review: The I-SPY 2 trial is an adaptive clinical trial platform designed to improve outcomes in high-risk breast cancer patients by testing new drugs in the neoadjuvant setting. The intent of this review is to discuss background, study structure, innovation, and outcomes of the I-SPY 2 trial.
Recent findings: I-SPY 2 evaluates new agents combined with standard therapy with pathologic complete response (pCR) as the primary endpoint. I-SPY-2 uses clinical biomarkers to classify breast cancer into 10 subtypes, with Bayesian adaptive randomization to allow individualized patient assignment to therapy arms to maximize treatment effects. A total of 7 drugs have graduated from I-SPY 2. Multiple new agents are currently in active enrollment in I-SPY 2.
Summary: I-SPY 2 uses an individualized approach in clinical trial design to improve high-risk breast cancer outcomes. The purpose of this review is to encourage further research and innovation in this area and bring more precise treatment options to breast cancer patients.
Keywords: Adaptive randomization; Breast cancer; I-SPY 2; Neoadjuvant chemotherapy; Pathological complete response.
Figures
References
-
- Oliver RT. Regarding: Valero V, Buzdar AU, Hortobagyi GN. Locally advanced breast cancer. The Oncologist 1996;1:8–17. Oncologist. 1996;1(4):278–9. - PubMed
-
- Fisher B, Jeong JH, Bryant J, Anderson S, Dignam J, Fisher ER, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364(9437):858–68. 10.1016/S0140-6736(04)16981-X. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials