LncRNA MALAT-1 promotes growth and metastasis of epithelial ovarian cancer via sponging microrna-22
- PMID: 33312345
- PMCID: PMC7724350
LncRNA MALAT-1 promotes growth and metastasis of epithelial ovarian cancer via sponging microrna-22
Abstract
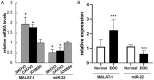
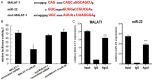
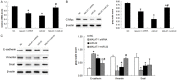
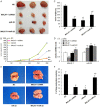
LncRNAs and miRNAs are emerging players in epithelial ovarian cancer (EOC). LncRNA MALAT-1 and miR-22 play vital roles in the onset and development of multiple cancers. Both of them are abnormally expressed in ovarian cancer, but the molecular basis for their involvement in EOC is unclear. In this study, we found MALAT-1 was up-regulated but miR-22 was down-regulated in EOC tissues and cell lines when compared to normal ovarian epithelial cell line IOSE80. Both of MALAT-1shRNA and miR-22 mimics inhibited ovarian cell proliferation, migration, and invasion, while simultaneously overexpressing MALAT-1 and miR-22 largely canceled out this inhibitory effect. Consistently, MALAT-1 silencing and miR-22 overexpression restrained tumor growth and metastasis to lungs in nude mice, which could be largely counteracted by co-overexpressing MALAT-1 and miR-22. Mechanistically, MALAT-1 targeted and sponged miR-22, counteracting its inhibitory effect on c-myc and c-myc-mediated epithelial-mesenchymal transition. Our findings for the first time demonstrated that MALAT-1 supports EOC progression through sponging miR-22, providing a novel insight into the role of MALAT-1 in ovarian cancer.
Keywords: Epithelial ovarian cancer; LncRNA MALAT-1; c-myc; miRNA-22.
AJTR Copyright © 2020.
Conflict of interest statement
None.
Figures

Similar articles
-
CircCERS6 Suppresses the Development of Epithelial Ovarian Cancer Through Mediating miR-630/RASSF8.Biochem Genet. 2022 Dec;60(6):2611-2629. doi: 10.1007/s10528-022-10227-2. Epub 2022 Jun 8. Biochem Genet. 2022. PMID: 35676548
-
lncRNA AC005224.4/miR-140-3p/SNAI2 regulating axis facilitates the invasion and metastasis of ovarian cancer through epithelial-mesenchymal transition.Chin Med J (Engl). 2023 May 5;136(9):1098-1110. doi: 10.1097/CM9.0000000000002201. Chin Med J (Engl). 2023. PMID: 36939239 Free PMC article.
-
LncRNA MALAT-1 competitively regulates miR-124 to promote EMT and development of non-small-cell lung cancer.Anticancer Drugs. 2018 Aug;29(7):628-636. doi: 10.1097/CAD.0000000000000626. Anticancer Drugs. 2018. PMID: 29782349
-
Silencing of lncRNA SNHG17 inhibits the tumorigenesis of epithelial ovarian cancer through regulation of miR-485-5p/AKT1 axis.Biochem Biophys Res Commun. 2022 Dec 31;637:117-126. doi: 10.1016/j.bbrc.2022.10.091. Epub 2022 Oct 29. Biochem Biophys Res Commun. 2022. PMID: 36399797
-
MALAT-1 Is a Key Regulator of Epithelial-Mesenchymal Transition in Cancer: A Potential Therapeutic Target for Metastasis.Cancers (Basel). 2024 Jan 4;16(1):234. doi: 10.3390/cancers16010234. Cancers (Basel). 2024. PMID: 38201661 Free PMC article. Review.
Cited by
-
lncRNA MALAT1 participates in metformin inhibiting the proliferation of breast cancer cell.J Cell Mol Med. 2021 Aug;25(15):7135-7145. doi: 10.1111/jcmm.16742. Epub 2021 Jun 24. J Cell Mol Med. 2021. PMID: 34164906 Free PMC article.
-
RAD51B-AS1 promotes the malignant biological behavior of ovarian cancer through upregulation of RAD51B.J Zhejiang Univ Sci B. 2024 Jul 15;25(7):581-593. doi: 10.1631/jzus.B2300154. J Zhejiang Univ Sci B. 2024. PMID: 39011678 Free PMC article.
-
Relationship between lncRNA MALAT1 and Chemo-radiotherapy Resistance of Cancer Cells: Uncovered Truths.Cell Biochem Biophys. 2024 Sep;82(3):1613-1627. doi: 10.1007/s12013-024-01317-6. Epub 2024 May 28. Cell Biochem Biophys. 2024. PMID: 38806965 Review.
-
Epigenetics in diabetic cardiomyopathy.Clin Epigenetics. 2024 Apr 5;16(1):52. doi: 10.1186/s13148-024-01667-1. Clin Epigenetics. 2024. PMID: 38581056 Free PMC article. Review.
-
LINC00704 facilitates cell proliferation, migration, and invasion via miR-323a-3p/SLC44A1 axis in epithelial ovarian cancer.Discov Oncol. 2025 Apr 29;16(1):640. doi: 10.1007/s12672-025-01866-z. Discov Oncol. 2025. PMID: 40301147 Free PMC article.
References
-
- Rupaimoole R, Lee J, Haemmerle M, Ling H, Previs RA, Pradeep S, Wu SY, Ivan C, Ferracin M, Dennison JB, Millward NMZ, Nagaraja AS, Gharpure KM, McGuire M, Sam N, Armaiz-Pena GN, Sadaoui NC, Rodriguez-Aguayo C, Calin GA, Drapkin RI, Kovacs J, Mills GB, Zhang W, Lopez-Berestein G, Bhattacharya PK, Sood AK. Long noncoding RNA ceruloplasmin promotes cancer growth by altering glycolysis. Cell Rep. 2015;13:2395–2402. - PMC - PubMed
-
- Zhao MY, Qiu Y, Yang B, Sun Li, Hei K, Du X, Li YM. Long non-coding RNAs involved in gynecological cancer. Int J Gynecol Cancer. 2014;24:1140–1145. - PubMed
-
- Ji P, Diederichs S, Wang WB, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Müller-Tidow C. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
