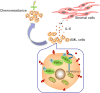
Stromal cells promote chemoresistance of acute myeloid leukemia cells via activation of the IL-6/STAT3/OXPHOS axis
- PMID: 33313091
- PMCID: PMC7723653
- DOI: 10.21037/atm-20-3191
Stromal cells promote chemoresistance of acute myeloid leukemia cells via activation of the IL-6/STAT3/OXPHOS axis
Abstract
Background: Bone marrow stromal cells (BMSCs) are known to promote chemoresistance in acute myeloid leukemia (AML) cells. However, the molecular basis for BMSC-associated AML chemoresistance remains largely unexplored.
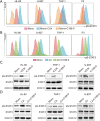
Methods: The mitochondrial oxidative phosphorylation (OXPHOS) levels of AML cells were measured by a Seahorse XFe24 cell metabolic analyzer. The activity of total or mitochondrial signal transducer and transcription activator 3 (STAT3) in AML cells was explored by flow cytometry and Western blotting. Real-time quantitative PCR, Western blotting and enzyme-linked immunosorbent assay (ELISA) were used to analyze expression of interleukin 6 (IL-6) in the human BMSC line HS-5, and IL-6 was knocked out in HS-5 cells by CRISPR/Cas9 system.
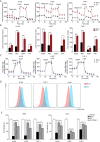
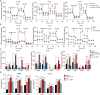
Results: In this study, we observed that co-culturing with BMSCs heightened OXPHOS levels in AML cells, thus promoting chemoresistance in these cells. HS-5 cell-induced upregulation of OXPHOS is dependent on the activation of STAT3, especially on that of mitochondrial serine phosphorylated STAT3 (pS-STAT3) in AML cells. The relationship among pS-STAT3, OXPHOS, and chemosensitivity of AML cells induced by BMSCs was demonstrated by the STAT3 activator and inhibitor, which upregulated and downregulated the levels of mitochondrial pS-STAT3 and OXPHOS, respectively. Intriguingly, AML cells remodeled HS-5 cells to secrete more IL-6, which augmented mitochondrial OXPHOS in AML cells and stimulated their chemoresistance. IL-6 knockout in HS-5 cells impaired the ability of these cells to activate STAT3, to increase OXPHOS, or to promote chemoresistance in AML cells.
Conclusions: BMSCs promoted chemoresistance in AML cells via the activation of the IL-6/STAT3/OXPHOS pathway. These findings exhibit a novel mechanism of chemoresistance in AML cells in the bone marrow microenvironment from a metabolic perspective.
Keywords: IL-6/STAT3/OXPHOS axis; acute myeloid leukemia (AML); chemosensitivity; stromal cells.
2020 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3191). The authors have no conflicts of interest to declare.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
