Automated Measurements of Key Morphological Features of Human Embryos for IVF
- PMID: 33313603
- PMCID: PMC7732604
- DOI: 10.1007/978-3-030-59722-1_3
Automated Measurements of Key Morphological Features of Human Embryos for IVF
Abstract
A major challenge in clinical In-Vitro Fertilization (IVF) is selecting the highest quality embryo to transfer to the patient in the hopes of achieving a pregnancy. Time-lapse microscopy provides clinicians with a wealth of information for selecting embryos. However, the resulting movies of embryos are currently analyzed manually, which is time consuming and subjective. Here, we automate feature extraction of time-lapse microscopy of human embryos with a machine-learning pipeline of five convolutional neural networks (CNNs). Our pipeline consists of (1) semantic segmentation of the regions of the embryo, (2) regression predictions of fragment severity, (3) classification of the developmental stage, and object instance segmentation of (4) cells and (5) pronuclei. Our approach greatly speeds up the measurement of quantitative, biologically relevant features that may aid in embryo selection.
Keywords: Deep Learning; Human Embryos; In-Vitro Fertilization.
Figures
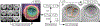
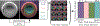
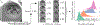

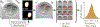

References
-
- Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C, Scott RT: Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertility and sterility 71(5), 836–842 (1999) - PubMed
-
- Bellman R: Dynamic programming. Science 153(3731), 34–37 (1966) - PubMed
-
- Broughton DE, Moley KH: Obesity and female infertility: potential mediators of obesity’s impact. Fertility and sterility 107(4), 840–847 (2017) - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
