Neuregulin-1 beta 1 is implicated in pathogenesis of multiple sclerosis
- PMID: 33313801
- PMCID: PMC7880664
- DOI: 10.1093/brain/awaa385
Neuregulin-1 beta 1 is implicated in pathogenesis of multiple sclerosis
Abstract
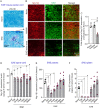
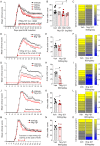
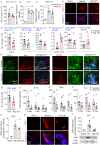
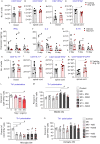
Multiple sclerosis is characterized by immune mediated neurodegeneration that results in progressive, life-long neurological and cognitive impairments. Yet, the endogenous mechanisms underlying multiple sclerosis pathophysiology are not fully understood. Here, we provide compelling evidence that associates dysregulation of neuregulin-1 beta 1 (Nrg-1β1) with multiple sclerosis pathogenesis and progression. In the experimental autoimmune encephalomyelitis model of multiple sclerosis, we demonstrate that Nrg-1β1 levels are abated within spinal cord lesions and peripherally in the plasma and spleen during presymptomatic, onset and progressive course of the disease. We demonstrate that plasma levels of Nrg-1β1 are also significantly reduced in individuals with early multiple sclerosis and is positively associated with progression to relapsing-remitting multiple sclerosis. The functional impact of Nrg-1β1 downregulation preceded disease onset and progression, and its systemic restoration was sufficient to delay experimental autoimmune encephalomyelitis symptoms and alleviate disease burden. Intriguingly, Nrg-1β1 therapy exhibited a desirable and extended therapeutic time window of efficacy when administered prophylactically, symptomatically, acutely or chronically. Using in vivo and in vitro assessments, we identified that Nrg-1β1 treatment mediates its beneficial effects in EAE by providing a more balanced immune response. Mechanistically, Nrg-1β1 moderated monocyte infiltration at the blood-CNS interface by attenuating chondroitin sulphate proteoglycans and MMP9. Moreover, Nrg-1β1 fostered a regulatory and reparative phenotype in macrophages, T helper type 1 (Th1) cells and microglia in the spinal cord lesions of EAE mice. Taken together, our new findings in multiple sclerosis and experimental autoimmune encephalomyelitis have uncovered a novel regulatory role for Nrg-1β1 early in the disease course and suggest its potential as a specific therapeutic target to ameliorate disease progression and severity.
Keywords: disease pathogenesis; experimental autoimmune encephalomyelitis; immune regulation; multiple sclerosis; neuregulin-1.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



Comment in
-
Neuregulin therapy for multiple sclerosis: an each-way bet?Brain. 2021 Feb 12;144(1):6-8. doi: 10.1093/brain/awaa434. Brain. 2021. PMID: 33578423 No abstract available.
References
-
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM.. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 2011; 14: 1142–9. - PubMed
-
- Alizadeh A, Dyck SM, Kataria H, Shahriary GM, Nguyen DH, Santhosh KT, et al.Neuregulin-1 positively modulates glial response and improves neurological recovery following traumatic spinal cord injury. Glia 2017; 65: 1152–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

