Abiraterone acetate in combination with androgen deprivation therapy compared to androgen deprivation therapy only for metastatic hormone-sensitive prostate cancer
- PMID: 33314020
- PMCID: PMC8092456
- DOI: 10.1002/14651858.CD013245.pub2
Abiraterone acetate in combination with androgen deprivation therapy compared to androgen deprivation therapy only for metastatic hormone-sensitive prostate cancer
Abstract
Background: Systemic androgen deprivation therapy (ADT), also referred to as hormone therapy,ÃÂ has long been the primary treatment for metastatic prostate cancer. Additional agents have been reserved for the castrate-resistant disease stage when ADT start becoming less effective. Abiraterone is an agent with an established role in that disease stage, which has only recently been evaluated in the hormone-sensitive setting.
Objectives: To assess the effects of early abiraterone acetate, in combination with systemic ADT, for newly diagnosed metastatic hormone-sensitive prostate cancer.
Search methods: We searched CENTRAL, MEDLINE, Embase, six other databases, two trials registries, grey literature, and conference proceedings, up to 15 May 2020. We applied no restrictions on publication language or status.
Selection criteria: We included randomized trials, in which men diagnosed with hormone-sensitive prostate cancer were administered abiraterone acetate and prednisolone with ADT or ADTÃÂ alone.
Data collection and analysis: Two review authors independently classified studies and abstracted data from the included studies. We performed statistical analyses using a random-effects model. We rated the quality of evidence according to the GRADE approach.
Main results: The search identified two randomized controlled trials (RCT), with 2201 men, who were assigned to receive either abiraterone acetate 1000 mg once daily and low dose prednisone (5mg) in addition to ADT, or ADT alone. In the LATITUDE trial, the median age and range of men in the intervention group was 68 (38 to 89) years, and 67 (33 to 92) years in the control group. Nearly all of the men in thisÃÂ study (97.6%) had prostate cancer with a Gleason score of at least 8 (ISUP grade group 4). Primary outcomes The addition of abiraterone acetate to ADT reduces the probability of death from any cause compared to ADT alone (hazard ratio [HR] 0.64, 95% confidence interval [CI] 0.56 to 0.73; 2 RCTs, 2201 men; high certainty of evidence); this corresponds to 163 fewer deaths per 1000 men with hormone-sensitive metastaticÃÂ prostate cancerÃÂ (210 fewer to 115 fewer) at five years. Abiraterone acetate in addition to ADT probably results in little to no differenceÃÂ in quality of life compared to ADT alone, measured with the Functional Assessment of Cancer Therapy-prostate total score (FACT-P; range 0 to 156; higher values indicates better quality of life),ÃÂ at 12 months (mean difference [MD] 2.90 points, 95% CI 0.11 to 5.60; 1 RCT, 838 men; moderate certainty of evidence). Secondary outcomes Abiraterone plus ADT increases the risk of grades III to V adverse events compared to ADT alone (risk ratio [RR] 1.34, 95% CI 1.22 to 1.47; 1 RCT, 1199 men; high certainty of evidence); this corresponds to 162 more grade III to VÃÂ events per 1000 men with hormone-sensitive metastaticÃÂ prostate cancerÃÂ (105 more to 224 more) at a median follow-up of 30ÃÂ months. Abiraterone acetate in addition to ADT probably reduces the probability of death due to prostate cancer compared to ADT alone (HR 0.58, 95% CI 0.50 to 0.68; 2 RCTs, 2201 men; moderate certainty of evidence). This corresponds to 120 fewer death from prostate cancer per 1000 men with hormone-sensitive metastaticÃÂ prostate cancerÃÂ (95% CI 145 fewer to 90 fewer) afterÃÂ a median follow-up of 30 months. The addition of abiraterone acetate to ADT probably decreases the probability of disease progression compared to ADT alone (HR 0.35, 95%CI 0.26 to 0.49; 2 RCTs, 2097 men; moderate certainty of evidence). This corresponds to 369 fewer incidences of disease progression per 1000 men with hormone-sensitive metastaticÃÂ prostate cancerÃÂ (456 fewer to 256 fewer)ÃÂ after a median follow-up of 30 months. The addition of abiraterone acetate to ADT probably increases the risk of discontinuing treatment due to adverse events compared to ADT alone (RR 1.50, 95% CI 1.17 to 1.92; 1 RCT, 1199 men; moderate certainty of evidence). This corresponds to 51 more men (95% CI 17 more to 93 more) discontinuing treatment because of adverse events per 1000 men treated with abiraterone acetate and ADT compared to ADT alone afterÃÂ a median follow-up of 30 months.
Authors' conclusions: The addition of abiraterone acetate to androgen deprivation therapy improves overall survival but probably not quality of life. ItÃÂ probably also extends disease-specific survival, and delays disease progression compared to androgen deprivation therapy alone. However, the risk of grades III to V adverse events is increased, and probably, so is the risk of discontinuing treatment due to adverse events.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
NS: none known
MO: none known
PD: none known
SB: none known
SG declares the following:
Stock and other ownership interests: Nektar
Honoraria: Janssen Oncology, AstraZeneca, Exelixis, Bristol Myers Squibb, Merck Sharp & Dohme, Seattle Genetics, Pfizer
Speakers' Bureau: Bristol Myers Squibb, Janssen Oncology, Exelixis
Research funding: Merck, Pfizer, Bristol Myers Squibb, Seattle Genetics
BK declares the following relationships: PhotoCure Inc, FKD Therapeutics, Bristol Myers Squibb, Taris Biomedical, Boston Scientific Group, Merck Inc, Francis Medical
FK: none known
Figures
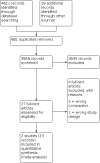













Update of
References
References to studies included in this review
Fizazi 2017 {published and unpublished data}
-
- CHi K, Protheroe A, Rodriguez Antolin A, Facchini G, Suttman H, Matsubara N, et al. Benefits of abiraterone acetate plus prednisone (AA1P) when added to androgen deprivation therapy (ADT) in LATITUDE on patient (Pt) reported outcomes (PRO). Annals of Oncology 2017;28(Suppl 5):v269. - PubMed
-
- CHi K, Tran N, Feyerabend S, Matsubara N, Ozguroglu M, Fein LE, et al. Subsequent treatment after abiraterone acetate + prednisone (AA + P) in patients (pts) with newly diagnosed high-risk metastatic castration-naïve prostate cancer (NDx-HR mCNPC): detailed analyses from the phase 3 LATITUDE trial. Journal of Clinical Oncology 2018;36(15 Suppl 1):5028.
-
- Chi KN, Protheroe A, Rodriguez-Antolin A, Facchini G, Suttman H, Matsubara N, et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncology 2018;19(2):194-206. - PubMed
-
- Fizazi K, Feyerabend S, Matsubara N, Ozguroglu M, Fein LE, Antolin AT. Longer term preplanned efficacy and safety analysis of abiraterone acetate plus prednisone (AA plus P) in patients (pts) with newly diagnosed high-risk metastatic castration-naive prostate cancer (NDx-HR mCNPC) from the phase 3 LATITUDE trial. Journal of Clinical Oncology 2018;36(15):5028.
James 2017 {published data only (unpublished sought but not used)}
-
- ClinicalTrialsgov. Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE). ClinicalTrials.gov 2005.
-
- Hoyle AP, Ali A, James ND, Cook A, Parker CC, Bono JS, et al. Abiraterone in "high-" and "low-risk" metastatic hormone-sensitive prostate cancer. European Urology 2019;76(6):719-28. - PubMed
-
- James ND, Bono JS, Spears MR, Clarke N, Mason MD, Dearnaley D, et al. Adding abiraterone for men with high-risk prostate cancer (PCa) starting longterm androgen deprivation therapy (ADT): survival results from STAMPEDE (NCT00268476). Journal of Clinical Oncology 2017;35(18 Suppl):LBA5003.
References to studies excluded from this review
Buelens 2018 {published data only}
-
- Buelens S, Poelaert F, Dhondt B, Fonteyne V, De Visschere P, Ost P, et al. Metastatic burden in newly diagnosed hormone-naive metastatic prostate cancer: comparing definitions of CHAARTED and LATITUDE trial. Urologic Oncology: Seminars and Original Investigations 2018;36(4):158. - PubMed
Feyerabend 2018 {published data only}
-
- Feyerabend S, Saad F, Li T, Ito T, Diels J, Sanden SV. Indirect treatment comparison (ITC) of abiraterone acetate (AA) plus prednisone (P) and docetaxel (DOC) on patient-reported outcomes (PROs) in metastatic castration-naive prostate cancer (mCNPC). Journal of Clinical Oncology 2018;36(Suppl 6):200.
Sydes 2017 {published data only}
-
- Sydes MR, Mason MD, Spears MR, Clarke NW, Dearnaley D, Ritchie AWS, et al. Adding abiraterone acetate plus prednisolone (AAP) or docetaxel for patients (pts) with high-risk prostate cancer (PCa) starting long-term androgen deprivation therapy (ADT): Directly randomised data from STAMPEDE (NCT00268476). Annals of Oncology 2017;28(Suppl 5):v619.
Additional references
Attard 2008
-
- Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. Journal of Clinical Oncology 2008;26(28):4563-71. - PubMed
Beer 2014
Berruti 2005
Cella 2009
-
- Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the functional assessment of cancer therapy – prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value in Health 2009;12(1):124-9. - PubMed
Chi 2019
-
- Chi KM, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. New England Journal of Medicine 2019;381:13-24. - PubMed
Davis 2019
-
- Davis ID, Martin AJ, Stockler MR, Begbie S, Kim KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. New England Journal of Medicine 2019;381:121-31. - PubMed
de Bono 2010
-
- Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al, TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376(9747):1147-54. - PubMed
de Bono 2011
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/cochrane-handbook.
EndNote 2016 [Computer program]
-
- EndNote. Version 7.5. Philadelphia (PA): Clarivate Analytics, 2016. Available at endnote.com.
Fizazi 2012
-
- Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncology 2012;13(10):983-92. - PubMed
Gillessen 2015
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed prior to 04 July 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), N/A. Available at gradepro.org.
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ, et al. What is "quality of evidence" and why is it important to clinicians? BMJ (Clinical Research Ed.) 2008;336(7651):995-8. [DOI: 10.1136/bmj.39490.551019.BE] - DOI - PMC - PubMed
Guyatt 2011
Hamdy 2016
-
- Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. New England Journal of Medicine 2016;375(15):1415-24. - PubMed
Heinlein 2004
-
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocrine Reviews 2004;25(2):276-308. - PubMed
Higgins 2002
Higgins 2003
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/cochrane-handbook.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/cochrane-handbook.
Huggins 1941
-
- Huggins C, Stevens RE Jr, Hodges C V. Studies on prostatic cancer: Ii. the effects of castration on advanced carcinoma of the prostate gland. Archives of Surgery 1941;43(2):209-23.
Inoue 2009
-
- Inoue T, Segawa T, Kamba T, Yoshimura K, Nakamura E, Nishiyama H, et al. Prevalence of skeletal complications and their impact on survival of hormone refractory prostate cancer patients in Japan. Urology 2009;73(5):1104-9. - PubMed
James 2015
-
- James ND, Spears MR, Clarke NW, Dearnaley DP, De Bono JS, Gale J, et al. Survival with newly diagnosed metastatic prostate cancer in the “Docetaxel Era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). European Urology 2015;67(6):1028-38. - PubMed
James 2016
-
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387(10024):1163-77. - PMC - PubMed
Janknegt 1997
-
- Janknegt RA, Boon TA, de Beek C, Grob P. Combined hormono/chemotherapy as primary treatment for metastatic prostate cancer: a randomized, multicenter study of orchiectomy alone versus orchiectomy plus estramustine phosphate. The Dutch Estracyt Study Group. Urology 1997;49(3):411-20. - PubMed
Kantoff 2010
-
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New England Journal of Medicine 2010;363(5):411-22. - PubMed
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] - DOI - PMC - PubMed
Millikan 2008
Murphy 1983
-
- Murphy GP, Beckley S, Brady MF, Ming Chu T, Dekernion JB, Dhabuwala C, et al. Treatment of newly diagnosed metastatic prostate cancer patients with chemotherapy agents in combination with hormones versus hormones alone. Cancer 1983;51(7):1264-72. - PubMed
Petrylak 2004
-
- Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. New England Journal of Medicine 2004;351(15):1513-20. - PubMed
Pummer 1997
-
- Pummer K, Lehnert M, Stettner H, Hubmer G. Randomized comparison of total androgen blockade alone versus combined with weekly epirubicin in advanced prostate cancer. European Urology 1997;32 Suppl 3:81-5. - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryan 2013
Ryan 2015
-
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncology 2015;16(2):152-60. - PubMed
Rydzewska 2017
Sathianathen 2017
Sathianathen 2018
Sathianathen 2019
-
- Sathianathen NJ, Koschel S, Thangasamy IA, Teh J, Alghazo O, Butcher G, et al. Indirect comparisons of efficacy between combination approaches in metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. European Urology 2019;77(3):365-72. [DOI: 10.1016/j.eururo.2019.09.004] [PMID: ] - DOI - PubMed
Sathianathen 2019a
-
- Sathianathen NJ, Alarid-Escudero F, Kuntz KM, Lawrentschuk N, Bolton DM, Kim SP et al. A cost-effectiveness analysis of systemic therapy for metastatic hormone-sensitive prostate cancer. European Urology Oncology 2019;2(6):649-55. - PubMed
Scher 2008
-
- Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. Journal of Clinical Oncology 2008;26(7):1148-59. - PMC - PubMed
Scher 2012
-
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New England Journal of Medicine 2012;367(13):1187-97. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/cochrane-handbook.
Siegel 2018
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a Cancer Journal for Clinicians 2018;68(1):7-30. - PubMed
Soifer 2012
Sweeney 2015
Tannock 2004
-
- Tannock IF, Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New England Journal of Medicine 2004;351(15):1502-12. - PubMed
Tilki 2016
-
- Tilki D, Schaeffer EM, Evans CP. Understanding mechanisms of resistance in metastatic castration-resistant prostate cancer: the role of the androgen receptor. European Urology Focus 2016;2(5):499-505. - PubMed
Torre 2015
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a Cancer Journal for Clinicians 2015;65(2):87-108. - PubMed
Vale 2018
Wallis 2018
-
- Wallis CJ, Klaassen Z, Bhindi B, Goldberg H, Chandrasekar T, Farrell AM, et al. Comparison of abiraterone acetate and docetaxel with androgen deprivation therapy in high-risk and metastatic hormone-naive prostate cancer: a systematic review and network meta-analysis. European Urology 2018;73(6):834-44. - PubMed
Zelefsky 2010
-
- Zelefsky MJ, Eastham JA, Cronin AM, Fuks Zi, Zhang Z, Yamada Y, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. Journal of Clinical Oncology 2010;28(9):1508-13. - PMC - PubMed
References to other published versions of this review
Sathianathen 2019b
-
- Sathianathen NJ, Oestreich MC, Brown SJane, Gupta S, Konety BR, Dahm P, Kunath F. Abiraterone acetate in combination with androgen deprivation therapy compared to androgen deprivation therapy only for metastatic hormone-sensitive prostate cancer. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD013245. [DOI: 10.1002/14651858.CD013245] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

