Systematic review with meta-analysis: safety and tolerability of immune checkpoint inhibitors in patients with pre-existing inflammatory bowel diseases
- PMID: 33314269
- PMCID: PMC8041067
- DOI: 10.1111/apt.16217
Systematic review with meta-analysis: safety and tolerability of immune checkpoint inhibitors in patients with pre-existing inflammatory bowel diseases
Abstract
Background: Immune checkpoint inhibitors may variably impact patients with pre-existing autoimmune diseases.
Aims: To evaluate the risks and outcomes of adverse events in patients with pre-existing inflammatory bowel diseases treated with immune checkpoint inhibitors.
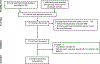
Methods: Through a systematic literature review up until July 31, 2020, we identified 12 studies reporting the impact of immune checkpoint inhibitors in 193 patients with inflammatory bowel disease. Outcomes of interest were relapse of inflammatory bowel disease, need for corticosteroids and/or biologics to manage inflammatory bowel disease relapse, and discontinuation of immune checkpoint inhibitors. We calculated pooled rates (with 95% confidence intervals [CI]) using random effects meta-analysis, and examined risk factors associated with adverse outcomes through qualitative synthesis of individual studies.
Results: On meta-analysis, 40% patients (95% CI, 26%-55%) experienced relapse of inflammatory bowel disease with immune checkpoint inhibitors. Among patients who experienced relapse, 76% (95% CI, 65%-85%) required corticosteroids, and 37% (95% CI, 22%-53%) required biologic therapy. Overall, 35% patients (95% CI, 17%-57%) with inflammatory bowel disease discontinued immune checkpoint inhibitors. Gastrointestinal perforation and abdominal surgery due to immune checkpoint inhibitor complications occurred in <5% patients. In a large study, inhibitors of cytotoxic T-lymphocyte antigen-4 (CTLA-4) were associated with a higher risk of relapse than programmed cell death 1 (PD-1) and programmed death ligand 1 (PD-L1) inhibitors.
Conclusions: Approximately 40% of patients with pre-existing inflammatory bowel diseases experience relapse with immune checkpoint inhibitors, with most relapsing patients requiring corticosteroids and one-third requiring biologics. CTLA-4 inhibitors may be associated with higher risk of relapse.
© 2020 John Wiley & Sons Ltd.
Figures

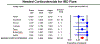


Comment in
-
Letter: safety of immune checkpoint inhibitors in patients with pre-established microscopic colitis-a single-centre experience.Aliment Pharmacol Ther. 2021 Jul;54(2):217-218. doi: 10.1111/apt.16458. Aliment Pharmacol Ther. 2021. PMID: 34170536 No abstract available.
References
-
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158–168. - PubMed
-
- Collins M, Soularue E, Marthey L, et al. Management of Patients With Immune Checkpoint Inhibitor-Induced Enterocolitis: A Systematic Review. Clin Gastroenterol Hepatol 2020;18:1393–1403.e1. - PubMed
-
- Abdel-Wahab N, Shah M, Lopez-Olivo MA, et al. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann Intern Med 2018;168:121–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
