The prognostic value of general laboratory testing in patients with COVID-19
- PMID: 33314316
- PMCID: PMC7883082
- DOI: 10.1002/jcla.23668
The prognostic value of general laboratory testing in patients with COVID-19
Abstract
Background: Lymphocyte count (LYM) of peripheral blood and some indices of general biochemical analysis had diagnostic and prognostic value for coronavirus disease 2019 (COVID-19), and the value of other remaining indices is rare.
Methods: A total of 94 patients with COVID-19 were enrolled at Renmin Hospital of Wuhan University. According to the severity of COVID-19, the patients were divided into three groups (moderate 49, severe 35, and critical 10), and 40 healthy cases were enrolled in the same period as healthy controls. The diagnostic and prognostic value of indices in peripheral blood cell count and general biochemical analysis was analyzed.
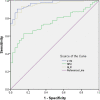
Results: Compared with healthy cases, the value differences in peripheral blood analysis in patients with COVID-19 were statistically significant (p < 0.01), the differences in LYM, neutrophil count (Neu), platelet count (PLT), and white blood cell count (WBC) were statistically significant among different severity of COVID-19 (p < 0.05). Compared with healthy cases, the differences in general biochemical results in patients with COVID-19 were statistically significant (p < 0.01), the value differences in direct bilirubin (DBIL), low-density lipoprotein cholesterol (LDL-Ch), and nitrogen (urea) were statistically significant among different severity of COVID-19 (p < 0.05). Neutrophil/lymphocyte ratio (NLR) had higher sensitivity and specificity for COVID-19 diagnosis.
Conclusions: Some indices of peripheral blood cell count and general biochemical analysis were valuable in discriminating COVID-19 and predicting severity and adverse outcome of patients with COVID-19. For clinician, it is better to use more economical and easy-to-get indices to diagnose and predict the prognosis of COVID-19.
Keywords: COVID-19; biochemical analysis; peripheral blood cell count; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
© 2020 The Authors. Journal of Clinical Laboratory Analysis published by Wiley Periodicals LLC.
Conflict of interest statement
We declare that we have no conflicts of interest.
Figures
References
-
- Coronavirus disease (COVID‐2019) situation reports . https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situatio.... Accessed 15 February 2020.
-
- General Office of the National Health Committee of China, China Traditional Chinese Medicine Administration Office . Diagnosis and treatment plan of novel coronavirus pneumonia (seventh trial edition). 2020. http://www.nhc.gov.cn/yzygj/s7652m/202003/a31191442e29474b98bfed5579d5af.... Accessed 11 March 2020.
-
- Scheller J, Chalaris A, Schmidt‐Arras D, Rose‐John S. The pro‐ and anti‐inflammatory properties of the cytokine interleukin‐6, BBA‐Mol. Cell Res. 2011;1813(5):878‐888. - PubMed
MeSH terms
Grants and funding
- 2017ZX10103005/National Mega Project on Major Infectious Disease Prevention
- PWR12018-05/Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai
- PWZxq2017-15/Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai
- 81672079/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous