Colors of Single-Wall Carbon Nanotubes
- PMID: 33314478
- PMCID: PMC11469110
- DOI: 10.1002/adma.202006395
Colors of Single-Wall Carbon Nanotubes
Abstract
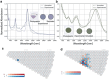
Although single-wall carbon nanotubes (SWCNTs) exhibit various colors in suspension, directly synthesized SWCNT films usually appear black. Recently, a unique one-step method for directly fabricating green and brown films has been developed. Such remarkable progress, however, has brought up several new questions. The coloration mechanism, potentially achievable colors, and color controllability of SWCNTs are unknown. Here, a quantitative model is reported that can predict the specific colors of SWCNT films and unambiguously identify the coloration mechanism. Using this model, colors of 466 different SWCNT species are calculated, which reveals a broad spectrum of potentially achievable colors of SWCNTs. The calculated colors are in excellent agreement with existing experimental data. Furthermore, the theory predicts the existence of many brilliantly colored SWCNT films, which are experimentally expected. This study shows that SWCNTs as a form of pure carbon, can display a full spectrum of vivid colors, which is expected to complement the general understanding of carbon materials.
Keywords: carbon nanotube thin films; color range; nanomaterials; pigments; quantitative coloration model.
© 2020 The Authors. Advanced Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Novoselov K. S., Science 2004, 306, 666. - PubMed
-
- Bonaccorso F., Sun Z., Hasan T., Ferrari A. C., Nat. Photonics 2010, 4, 611.
-
- Iijima S., Nature 1991, 354, 56.
-
- Saito R., Dresselhaus G., Dresselhaus M. S., Physical Properties of Carbon Nanotubes, Imperial College Press, London, UK: 1998.
-
- Yang Z., Ci L., Bur J. A., Lin S., Ajayan P. M., Nano Lett. 2008, 8, 446. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

