Diagnosis of Progressive Multiple Sclerosis From the Imaging Perspective: A Review
- PMID: 33315071
- PMCID: PMC11382596
- DOI: 10.1001/jamaneurol.2020.4689
Diagnosis of Progressive Multiple Sclerosis From the Imaging Perspective: A Review
Abstract
Importance: Although magnetic resonance imaging (MRI) is useful for monitoring disease dissemination in space and over time and excluding multiple sclerosis (MS) mimics, there has been less application of MRI to progressive MS, including diagnosing primary progressive (PP) MS and identifying patients with relapsing-remitting (RR) MS who are at risk of developing secondary progressive (SP) MS. This review addresses clinical application of MRI for both diagnosis and prognosis of progressive MS.
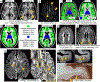
Observations: Although nonspecific, some spinal cord imaging features (diffuse abnormalities and lesions involving gray matter [GM] and ≥2 white matter columns) are typical of PPMS. In patients with PPMS and those with relapse-onset MS, location of lesions in critical central nervous system regions (spinal cord, infratentorial regions, and GM) and MRI-detected high inflammatory activity in the first years after diagnosis are risk factors for long-term disability and future progressive disease course. These measures are evaluable in clinical practice. In patients with established MS, GM involvement and neurodegeneration are associated with accelerated clinical worsening. Subpial demyelination and slowly expanding lesions are novel indicators of progressive MS.
Conclusions and relevance: Diagnosis of PPMS is more challenging than diagnosis of RRMS. No qualitative clinical, immunological, histopathological, or neuroimaging features differentiate PPMS and SPMS; both are characterized by imaging findings reflecting neurodegeneration and are also impacted by aging and comorbidities. Unmet diagnostic needs include identification of MRI markers capable of distinguishing PPMS from RRMS and predicting the evolution of RRMS to SPMS. Integration of multiple parameters will likely be essential to achieve these aims.
Conflict of interest statement
Conflict of interest disclosures
Figures
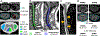
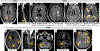
References
-
- Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. - PubMed
-
- Faissner S, Plemel JR, Gold R, Yong VW. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov. 2019. - PubMed
-
- Kalincik T, Cutter G, Spelman T, et al. Defining reliable disability outcomes in multiple sclerosis. Brain. 2015;138(Pt 11):3287–3298. - PubMed
-
- Lorscheider J, Buzzard K, Jokubaitis V, et al. Defining secondary progressive multiple sclerosis. Brain. 2016;139(Pt 9):2395–2405. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

