Mitophagy-mediated adipose inflammation contributes to type 2 diabetes with hepatic insulin resistance
- PMID: 33315085
- PMCID: PMC7927432
- DOI: 10.1084/jem.20201416
Mitophagy-mediated adipose inflammation contributes to type 2 diabetes with hepatic insulin resistance
Erratum in
-
Correction: Mitophagy-mediated adipose inflammation contributes to type 2 diabetes with hepatic insulin resistance.J Exp Med. 2025 Jul 7;222(7):e2020141606022025c. doi: 10.1084/jem.2020141606022025c. Epub 2025 Jun 13. J Exp Med. 2025. PMID: 40513072 Free PMC article. No abstract available.
Abstract
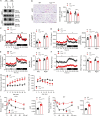
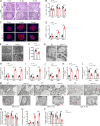
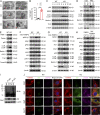
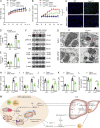
White adipose tissues (WAT) play crucial roles in maintaining whole-body energy homeostasis, and their dysfunction can contribute to hepatic insulin resistance and type 2 diabetes mellitus (T2DM). However, the mechanisms underlying these alterations remain unknown. By analyzing the transcriptome landscape in human adipocytes based on available RNA-seq datasets from lean, obese, and T2DM patients, we reveal elevated mitochondrial reactive oxygen species (ROS) pathway and NF-κB signaling with altered fatty acid metabolism in T2DM adipocytes. Mice with adipose-specific deletion of mitochondrial redox Trx2 develop hyperglycemia, hepatic insulin resistance, and hepatic steatosis. Trx2-deficient WAT exhibited excessive mitophagy, increased inflammation, and lipolysis. Mechanistically, mitophagy was induced through increasing ROS generation and NF-κB-dependent accumulation of autophagy receptor p62/SQSTM1, which recruits damaged mitochondria with polyubiquitin chains. Importantly, administration of ROS scavenger or NF-κB inhibitor ameliorates glucose and lipid metabolic disorders and T2DM progression in mice. Taken together, this study reveals a previously unrecognized mechanism linking mitophagy-mediated adipose inflammation to T2DM with hepatic insulin resistance.
© 2020 He et al.
Conflict of interest statement
Disclosures: G. Shulman reported grants from NIH (R01 DK119668, R01 DK116774, R01 DK113984, R01 DK114793, RC2 DK120534, and P30 DK045735), Gilead Sciences, Inc., AstraZeneca, and Merck & Co., Inc.; non-financial support from Gilead Sciences, Inc., AstraZeneca, Merck & Co., Inc., Janssen Research & Development, and Novo Nordisk; and personal fees from Gilead Sciences, Inc., AstraZeneca, Merck & Co., Inc., Janssen Research & Development, Novo Nordisk, iMetabolic Biopharma Corp., Maze Therapeutics, Inc., Generian Pharmaceuticals, Ionis Pharmaceuticals, Inc., BridgeBio, Esperion, 89Bio, Inc., Nimbus Discovery, Inc., Staten Biotechnology, Longitude Capital, Celgene Corp., and Aegerion Pharmaceuticals during the conduct of the study. No other disclosures were reported.
Figures










References
-
- Baixauli, F., Martin-Cofreces N.B., Morlino G., Carrasco Y.R., Calabia-Linares C., Veiga E., Serrador J.M., and Sanchez-Madrid F.. 2011. The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J. 30:1238–1250. 10.1038/emboj.2011.25 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

