Hospital Length of Stay for Patients with Severe COVID-19: Implications for Remdesivir's Value
- PMID: 33315210
- PMCID: PMC7734451
- DOI: 10.1007/s41669-020-00243-6
Hospital Length of Stay for Patients with Severe COVID-19: Implications for Remdesivir's Value
Conflict of interest statement
Dr. Anderson has no conflicts of interest that are directly relevant to the content of this article. Dr. Baldwin served as site co-investigator for Gilead Sciences Remdesivir trials GS-US-540-5774 and GS-US-540-5773 and received no funding. Dr. Bach has received personal fees and non-financial support from the American Society for Health-System Pharmacists, United Rheumatology, Oppenheimer & Co, Kaiser Permanente Institute for Health Policy, the Congressional Budget Office, America’s Health Insurance Plans, and Geisinger; personal fees from WebMD, Defined Health, JMP Securities, Mercer, Foundation Medicine, Grail, Morgan Stanley, NYS Rheumatology Society, Cello Health, Anthem, Magellan Health, EQRx, Meyer Cancer Center of Weill Cornell Medicine, and the National Pharmaceutical Council; personal fees, non-financial support, and other support from Oncology Analytics; and grants from Kaiser Permanente and Arnold Ventures. All listed support was outside the submitted work.
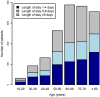
Figures
Update of
-
Hospital length of stay for severe COVID-19: implications for Remdesivir's value.medRxiv [Preprint]. 2020 Aug 12:2020.08.10.20171637. doi: 10.1101/2020.08.10.20171637. medRxiv. 2020. Update in: Pharmacoecon Open. 2021 Mar;5(1):129-131. doi: 10.1007/s41669-020-00243-6. PMID: 32817960 Free PMC article. Updated. Preprint.
References
-
- O'Day D. An open letter from Daniel O'Day, chairman and CEO, Gilead sciences. Forst City: Business Wirie; 2020.
-
- Coronavirus (COVID-19) Update: FDA issues emergency use authorization for potential COVID-19 treatment. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19.... Accessed 17 Sep 2020.
Grants and funding
LinkOut - more resources
Full Text Sources