Predictors of immunotherapy benefit in Merkel cell carcinoma
- PMID: 33315984
- PMCID: PMC7720777
- DOI: 10.18632/oncotarget.27823
Predictors of immunotherapy benefit in Merkel cell carcinoma
Abstract
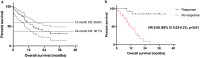
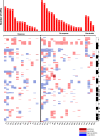
Merkel cell carcinoma is a rare cancer for which immune checkpoint blockade is standard-of-care for recurrent/metastatic disease. However, not all patients benefit from immunotherapy. A greater understanding of molecular mechanisms and predictive biomarkers are unmet needs. We retrospectively analyzed electronic health records and next-generation sequencing data of 45 patients treated at our institution from 2013 to 2020 to understand clinical and genomic correlates of benefit from immunotherapy. Our cohort predominantly included individuals with stage III disease at primary disease diagnosis and individuals with stage IV disease at recurrent/metastatic disease diagnosis. Most received immunotherapy as first-line treatment. 43% experienced objective response (median duration of response 24.2 months, 95% confidence interval 8.8-not reached). Median overall survival was 15.5 months (95% confidence interval 9.0-28.7) (median follow-up 25.2 months). Less advanced stage at primary disease diagnosis and shorter disease-free interval between completion of initial treatment and recurrence were each associated with greater odds of response (odds ratio of 0.06, p = 0.04 for stage; odds ratio 0.75, p = 0.05 for disease-free interval). Single-nucleotide variants in ARID2 and NTRK1 were associated with response (p = 0.05, without Bonferroni correction), while none of Merkel cell polyomavirus status, total mutational burden, ultraviolet mutational signatures, and copy-number alterations predicted outcomes. Patients with shorter disease-free interval may be particularly suitable immunotherapy candidates. Our molecular findings point to ARID2 and NTRK1 as potential predictive markers and/or therapeutic targets (e.g., with Trk inhibitors), although this association needs to be confirmed in a larger sample.
Keywords: Merkel cell carcinoma; cancer; genomics; immunotherapy; precision medicine.
Copyright: © 2020 Kacew et al.
Conflict of interest statement
CONFLICTS OF INTEREST AJK receives research support from the Pritzker School of Medicine and from the American Society of Hematology. Dr. Silk has received research funding (to the institution) from Biohaven Pharmaceuticals, Merck, Clinagen, and consulting fees from Bristol-Meyers Squibb, EMD Serono, Merck, and Sanofi Genzyme. JAD has received honoraria for advisory board participation with Merck & Co., Inc. and EMD Serono, Inc. JAD has received research funding from Constellation Pharmaceuticals, Inc. GJH has received research funding to institution from BMS, Exicure, GSK, Regeneron, Sanofi Genzyme, Kartos; consulting/honoraria from Regeneron, Sanofi Genzyme, BMS, Maverick, Merck, Kura, Bicara, and Exicure.
Figures

References
-
- Paulson KG, Park SY, Vandeven NA, Lachance K, Thomas H, Chapuis AG, Harms KL, Thompson JA, Bhatia S, Stang A, Nghiem P. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018; 78:457–63.e2. 10.1016/j.jaad.2017.10.028. - DOI - PMC - PubMed
-
- Kaufman HL, Russell JS, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, Shih KC, Lebbe C, Milella M, Brownell I, Lewis KD, Lorch JH, von Heydebreck A, et al.. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after >/=1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018; 6:7. 10.1186/s40425-017-0310-x. - DOI - PMC - PubMed
-
- Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Brohl AS, Friedlander PA, Daud A, Kluger HM, Reddy SA, Boulmay BC, Riker AI, Burgess MA, et al.. Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. J Clin Oncol. 2019; 37:693–702. 10.1200/JCO.18.01896. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

