Tubular mitochondrial AKT1 is activated during ischemia reperfusion injury and has a critical role in predisposition to chronic kidney disease
- PMID: 33316281
- PMCID: PMC7987863
- DOI: 10.1016/j.kint.2020.10.038
Tubular mitochondrial AKT1 is activated during ischemia reperfusion injury and has a critical role in predisposition to chronic kidney disease
Abstract
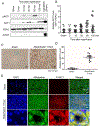
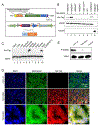
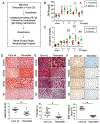
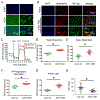
Kidney tubular dysfunction contributes to acute kidney injury and to the transition to chronic kidney disease. Although tubular mitochondria have been implicated in the pathophysiology of kidney failure, the mechanisms are not yet clear. Here, we demonstrated that ischemia-reperfusion injury induced acute translocation and activation of mitochondrial protein kinase B (also known as AKT1) in the kidney tubules. We hypothesized that mitochondrial AKT1 signaling protects against the development of acute kidney injury and subsequent chronic kidney disease. To test this prediction, we generated two novel kidney tubule-specific transgenic mouse strains with inducible expression of mitochondria-targeted dominant negative AKT1 or constitutively active AKT1, using a Cre-Lox strategy. Inhibition of mitochondrial AKT1 in mitochondria-targeted dominant negative AKT1 mice aggravated azotemia, tubular injuries, kidney fibrosis, glomerulosclerosis, and negatively impacted survival after ischemia-reperfusion injury. Conversely, enhancing tubular mitochondrial AKT1 signaling in mitochondria-targeted constitutively active AKT1 mice attenuated kidney injuries, protected kidney function, and significantly improved survival after ischemia-reperfusion injury (76.9% vs. 20.8%, respectively). Uncoupled mitochondrial respiration and increased oxidative stress was found in the kidney tubules when mitochondria AKT1 was inhibited, supporting the role of mitochondrial dysfunction in the pathophysiology of kidney failure. Thus, our studies suggest tubular mitochondrial AKT1 signaling could be a novel target to develop new strategies for better prevention and treatment of kidney injury.
Keywords: acute kidney injury; chronic kidney disease; ischemia refusion injury; mitochondrial AKT1.
Copyright © 2020 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

